Abstract
Background. The Charlson comorbidity index (CCI) has been considered as a valid and reliable tool for predicting poor clinical outcomes and mortality in patients with coronavirus disease 2019 (COVID-19). However, its relationship with the severity of pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has not been thoroughly explored.
Objectives. The aim of the present study was to identify the impact of the comorbidity burden, quantitatively assessed by applying CCI, on the severity of inpatient community-acquired pneumonia (CAP) caused by SARS-CoV-2.
Material and methods. The study was conducted using the medical records of 208 patients with CAP who had an epidemiological history of a plausible SARS-CoV-2 infection, with positive polymerase chain reaction (PCR) confirmation no later than 1 month before being admitted for inpatient treatment. The CCI was calculated using a custom computer program. The statistical analysis of data was carried out using Statistica, v. 7.0.
Results. Our study found a significant correlation between the comorbidity burden and the severity of CAP caused by SARS-CoV-2. Specifically, we observed a low CCI score in the majority of patients in the pneumonia risk class II and III groups, and a high CCI score ≥3 in the majority of patients in the pneumonia risk class IV group. Moreover, a direct correlation between CCI and age was established. The comorbidities most commonly associated with CAP caused by SARS-CoV-2 were congestive heart failure, moderate to severe liver diseases and diabetes mellitus (DM) with chronic complications.
Conclusions. The use of CCI to evaluate comorbid pathology in hospitalized patients with CAP caused by SARS-CoV-2 can assist the medical staff in developing timely preventive and therapeutic strategies, leading to improved patient prognosis.
Keywords: pneumonia, severity, Charlson comorbidity index, SARS-CoV-2
Introduction
The emergence of a new zoonotic infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease it causes – coronavirus disease 2019 (COVID-19) – posed an extraordinary challenge to the healthcare systems worldwide.1 On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic due to its rapid spread, ways of transmission and health risk.2 By April 11, 2023, WHO reported 685,044,510 cases of COVID-19, resulting in 6,838,093 deaths globally. By the same date, the number of confirmed cases of COVID-19 in Ukraine had reached 5,465,954, with 111,676 of these cases being lethal.3
Coronavirus disease 2019 is characterized by a range of heterogeneous clinical signs and symptoms, including nasal congestion, rhinorrhea, a sore throat, a cough, myalgia or fatigue, vomiting, fever, and headache, especially in patients with respiratory involvement.4, 5 While nearly 80% of patients are either asymptomatic or present with mild symptoms of acute respiratory infection (ARI),6 some patients may rapidly develop pneumonia and severe dyspnea associated with the acute respiratory distress syndrome (ARDS).7, 8, 9 Since the SARS-CoV-2 viral spike protein binds to the human angiotensin-converting enzyme 2 (ACE2) receptor, a high expression of this protein in the pulmonary tissue is one of the suggested mechanisms underlying severe lung damage.10 For instance, alveolar epithelial type II (ATII) cells, being more susceptible to SARS-CoV-2, undergo apoptosis when infected.11 These cells are involved in surfactant secretion. The destruction of pneumocytes leads to decreased surfactant levels in the alveoli, causing them to collapse, and resulting in pneumonia and ARDS.12 One of the hallmarks of SARS-CoV-2 pneumonia evolving to ARDS, which further disrupts ACE signaling pathways, is a cytokine storm that leads to systemic inflammation, coagulation disorders, endothelial damage, and the dysregulation of the immune system.13
Old age and comorbidities are the main risk factors for the development of pneumonia and ARDS in COVID-19 patients.14, 15, 16, 17 Physiological changes and diseases affecting metabolism and/or immunity also may aggravate the course of SARS-CoV-2 infection.18, 19, 20 When exposed to equivalent amounts of viral particles, patients with comorbidities develop an increased viral load as compared to those without comorbidities. Age is another factor that correlates with the severity of SARS-CoV-2-induced pneumonia.19, 21 This could be attributed to the age-related decline in the effectiveness of T cells and B cells, as well as the overproduction of type 2 cytokines, which may result in a reduced control of viral replication and a prolonged proinflammatory response, potentially leading to poor clinical outcomes in the elderly.21, 22
The Charlson comorbidity index (CCI) was introduced in 1987 as a standardized score to estimate the likelihood of death in various medical situations, taking into account the impact of the coexisting medical conditions on the outcome.19, 23 Even though CCI has been shown to be a valid and reliable tool for predicting poor clinical outcomes and mortality in patients with COVID-19,24 its association with the severity of pneumonia caused by SARS-CoV-2 has not been widely explored.
Therefore, the present study aimed to identify the impact of the comorbidity burden, quantitatively assessed by applying CCI, on the severity of inpatient community-acquired pneumonia (CAP) caused by SARS-CoV-2.
Material and methods
This non-interventional, single case–cohort study was conducted retrospectively using the medical records of 208 patients consecutively admitted for the treatment of CAP to the Department of Pulmonology of Ternopil Regional Clinical Hospital, Ukraine, from mid-January to the end of April 2021. At the time of admission, all patients tested negative for SARS-CoV-2 (the swab test).
The most common scoring systems used to predict severity and mortality in the case of CAP are CURB-65 (confusion, urea, respiratory rate, blood pressure (BP), age ≥65 years), SMART-COP (systolic blood pressure, multilobar infiltrates, albumin, respiratory rate, tachycardia, confusion, oxygen, and pH) and the pneumonia severity index (PSI), with the latter being originally developed within the Pneumonia Patient Outcomes Research Team (PORT) project. The PORT score determines PSI, predicts the risk of death and provides recommendations for efficient treatment. According to the total scoring of the disease signs (prognostic criteria), 5 classes of an increased risk of mortality are distinguished: class I: age <50 years, no comorbidity, no abnormal vital signs, <50 points; class II: 51–70 points; class III: 71–90 points; class IV: 91–130 points; and class V: >130 points.25
The diagnoses of CAP and the distribution of patients into risk classes according to the PORT scores were confirmed using the 2019 Ukrainian evidence-based clinical guidelines entitled “Nosocomial pneumonia in adults: Etiology, pathogenesis, classification, diagnostics, antimicrobial therapy, and prevention”,26 adapted from the National Institute for Health and Care Excellence (NICE) Clinical Guideline CG191 – Pneumonia in adults: Diagnosis and management.27
Based on the medical records, the patients were categorized into 3 groups according to their PORT scores: group II – patients with pneumonia of risk class II (n = 124); group III – patients with pneumonia of risk class III (n = 68); and group IV – patients with pneumonia of risk class IV (n = 16).
The study inclusion criteria were as follows: a positive swab test for SARS-CoV-2 no later than 1 month before being admitted for inpatient treatment; the presence of ARI symptoms; and the evidence of CAP on a high-resolution computed tomography (CT) scan. Patients under the age of 18, and pregnant or lactating women were excluded from the study.
A comparison group (CG, group I; n = 27) comprised patients admitted to the Department of Pulmonology of Ternopil Regional Clinical Hospital during the same period, who had a positive swab test for SARS-CoV-2 no later than 1 month before the admission for inpatient treatment, the symptoms of ARI and no evidence of pneumonia on a high-resolution CT scan.
The CCI was calculated using a custom computer program and the following input data: the age-based score starting at ≥50 years, with an increase of 1 point for every 10 years; a history of definitive or probable myocardial infarction (+1 point); congestive heart failure (+1 point); peripheral vascular disease (+1 point); cerebrovascular disease (+1 point); dementia (+1 point); chronic obstructive pulmonary disease (COPD) (+1 point); connective tissue disease (+1 point); peptic ulcer disease (+1 point); a liver disease (mild: +1 point; moderate to severe: +3 points); diabetes mellitus (DM) (+1 point; with chronic complications: +2 points); hemiplegia (+2 points); moderate to severe chronic kidney disease (CKD) (+2 points); a solid tumor (localized: +2 points; with metastases: +6 points); leukemia (+2 points); malignant lymphoma (+2 points); and the acquired immunodeficiency syndrome (AIDS) (+6 points). A CCI score ≥3 was considered diagnostically significant.28, 29
The data was collected by one researcher, and then verified by a second researcher.
Statistical analysis
Statistical analysis was performed using Statistica, v. 7.0 (StatSoft Inc., Tulsa, USA). The normality of quantitative variables was assessed using the Kolmogorov–Smirnov test. The variables were described as mean and standard deviation (M ±SD) or as number and percentage (n (%)). The comparative analysis of absolute values was conducted using the parametric analysis of variance (ANOVA) test. Pearson’s test was used for the comparison of relative values, which were presented as a percentage ratio. The χ2 test and Fisher’s exact test were used to compare the variables between the groups. Correlations were calculated using the Pearson correlation coefficient (r). Differences were considered statistically significant at a p-value <0.05.
Results
The mean patient age did not significantly differ between the study groups and CG (Table 1). At the same time, the median age of patients with CAP caused by SARS-CoV-2 was 59 years. Approximately 42% of the study population were 65 years of age or older.
The retrospective analysis of the data showed no significant differences in the severity of CAP caused by SARS-CoV-2 between male and female patients (Table 2).
The CCI revealed significant differences among the inpatient groups with CAP caused by SARS-CoV-2 in terms of risk classes, indicating a higher comorbidity burden in subjects with a higher risk class. The comparison of CCI between inpatients with CAP caused by SARS-CoV-2 and CG showed significantly higher scores in subjects with pneumonia risk class III (by 57.89%) and risk class IV (by 167.67%) (Table 3).
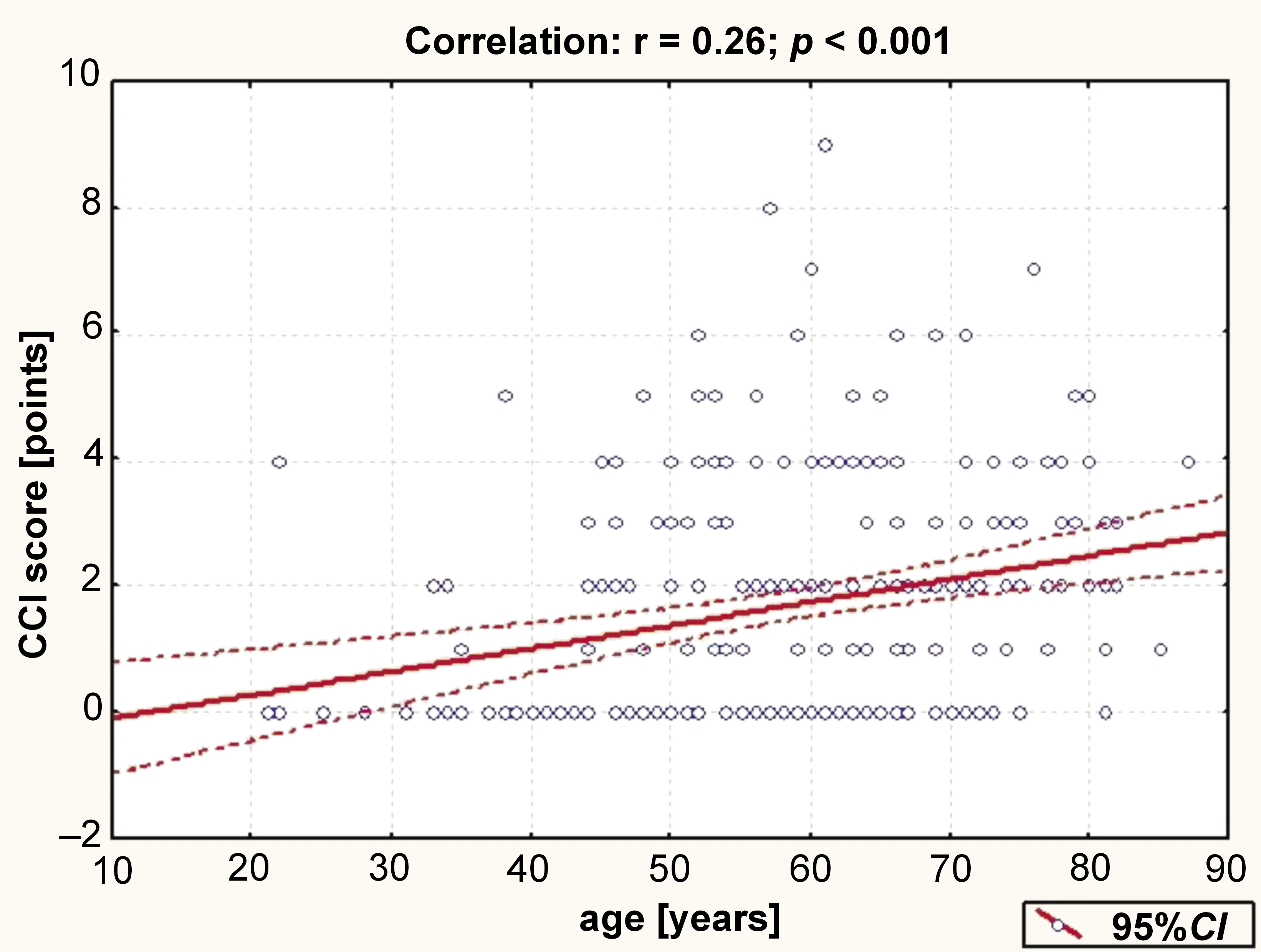
A weak direct correlation was found between the patients’ CCI score and age (Figure 1).
The severity of comorbid diseases was recorded and scored using the CCI formula. A cut-off value of 3 points was a substantial independent risk factor in predicting mortality and an indicator of a high comorbidity burden, while a ССІ score of 0–2 points indicated a low comorbidity burden. The study established a significant correlation between the comorbidity burden and the severity of pneumonia. In particular, patients in the pneumonia risk class II and III groups had a low CCI score, while those in group IV had a high CCI score. It is worth noting that among patients with a low CCI score, there were 31.99% more individuals in the pneumonia risk class III group than in the pneumonia risk class IV group (Table 4).
The analysis of the frequency of concomitant pathology, taking into account the severity of CAP caused by SARS-CoV-2, revealed significant relationships between the diagnosed cases of congestive heart failure, moderate to severe liver diseases and DM with chronic complications and the pneumonia risk class. In group IV, the frequency of diagnosed congestive heart failure, moderate to severe liver diseases and DM with chronic complications was significantly higher as compared to patients from group III (by 29.04%, 15.07% and 19.49%, respectively) and group II (by 37.30%, 27.22% and 24.80%, respectively) (Table 5).
Discussion
COVID-19 patients with multiple comorbidities are at a higher risk of poorer outcomes and mortality due to the increased severity of lung injury. Guan et al. evaluated the risk of serious adverse outcomes in COVID-19 patients by stratifying their comorbidity status, and showed that subjects with underlying diseases had poorer clinical outcomes than those without.30 Huang et al. demonstrated that 32% of hospitalized patients with pneumonia caused by SARS-CoV-2 had underlying diseases, including DM (20%), hypertension (HTN) (15%) and cardiovascular disease (CVD) (15%).31 Chen et al. reported that 51% of inpatients with pneumonia caused by SARS-CoV-2 had comorbidities, with the most prevalent being cardiovascular and cerebrovascular diseases (40%) and DM (13%).32 A prospective observational cohort study using the International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) WHO Clinical Characterisation Protocol UK (CCP-UK) found that chronic cardiac disease (30.9%), DM without complications (20.7%), COPD excluding asthma (17.7%), CKD (16.2%), and asthma (14.5%) were the most common major comorbidities among 20,133 hospital inpatients with COVID-19, while 22.5% of patients had no documented major underlying disease.33 In a retrospective study of 1,590 COVID-19 patients who were admitted to hospital and had a laboratory-confirmed diagnosis, 25.1% reported having at least one underlying health condition.30 Among these comorbidities, the most prevalent one was HTN (16.9%), followed by DM (8.2%) and other CVD (3.7%). Furthermore, individuals with severe COVID-19 were more likely to have multiple comorbidities as compared to non-severe cases (40.0% vs. 29.4%). Patients with multiple comorbidities were older (mean age: 66.2 years vs. 58.2 years) and were more likely to experience shortness of breath (55.4% vs. 34.1%).30 Wu et al. conducted a retrospective cohort study involving 201 patients with COVID-19 pneumonia, of which 84 (41.8%) developed ARDS.34 The study found that older age, neutrophilia, organ dysfunction, and elevated D-dimer levels were associated with an increased risk of ARDS or death. Moreover, among patients who developed ARDS, comorbidities such as HTN (23 out of 84 (27.4%)) and DM (16 out of 84 (19.0%)) were more common as compared to individuals who did not develop ARDS (16 out of 117 (13.7%) and 6 out of 117 (5.1%) patients, respectively).34 In their multicenter retrospective cohort study involving 191 patients with COVID-19 pneumonia, Zhou et al. found that older age, higher Sequential Organ Failure Assessment (SOFA) scores and D-dimer levels greater than 1 µg/mL upon admission were associated with an increased risk of death during hospitalization.22
Our results indicate that patients with comorbidities experienced more severe pneumonia as compared to those without comorbidities. Patients in the risk class IV group had a high CCI score (≥3), while the majority of patients in the risk class II and III groups had a low CCI score (0–2). The comorbidities most commonly associated with CAP caused by SARS-CoV-2 were congestive heart failure, moderate to severe liver diseases and diabetes with chronic complications. In group IV, the frequency of congestive heart failure, moderate to severe liver diseases and DM with chronic complications was significantly higher as compared to patients from group III (by 29.04%, 15.07% and 19.49%, respectively) and group II (by 37.30%, 27.22% and 24.80%, respectively).
Diabetes mellitus is a major risk factor for the development of severe pneumonia caused by viral infections.35 A single-center prospective cohort study found a strong association between DM and the diagnosis of pneumonia in patients with SARS-CoV-2 infection.36 The study observed that individuals with DM had a 3.5-fold higher prevalence of pneumonia as compared to those without the condition.36 According to Bornstein et al., patients with DM have up to a 50% higher risk of a fatal outcome from COVID-19 than those without DM.37 In a study by Zhu et al., patients with type 2 DM were more likely to experience a severe course of COVID-19, and those with poorer blood glucose control had a higher mortality rate than those with better blood glucose management.38
Among other comorbidities, congestive heart failure has also been associated with poor pneumonia outcomes due to alveolar flooding and reduced microbial clearance.39 A prospective study conducted by Petrilli et al. showed that chronic heart failure was associated with a 4.4-fold higher risk of hospitalization and a 1.9-fold higher risk of critical illness in COVID-19 patients.40 However, a study conducted by Bruno et al. examined the impact of the pre-existing chronic heart failure on the clinical outcomes of critically ill elderly patients (≥70 years) receiving intensive care for COVID-19, and concluded that the pre-existing condition did not influence 30-day mortality.41
Due to immune dysregulation, chronic liver diseases are believed to put affected individuals at risk of adverse outcomes following infection with SARS-CoV-2. Recent reports have indicated that patients with the pre-existing chronic liver diseases have a high mortality rate for COVID-19, with an associated mortality rate of 39.8%.42 According to a study by Marjot et al., the stage of liver disease is strongly correlated with COVID-19 mortality.43
We found a CCI score ≥3 in the majority of patients in the risk class IV group. It should be noted that a CCI score >3 (OR (odds ratio): 2.71; 95% CI (confidence interval): 1.85–3.97) was found to be independently associated with mortality in COVID-19 inpatients.44 Gregoriano et al. reported a significant burden of comorbidities among adult COVID-19 patients hospitalized in Aarau Cantonal Hospital, Switzerland, between February 26, 2020, and April 30, 2020.45 The patients had a median CCI of 3 points, and there was a high prevalence of HTN, CKD and obesity.45 In a systematic review and meta-analysis conducted by Tuty Kuswardhani et al., it was found that a CCI score of 1–2 or ≥3 was prognostically associated with higher mortality and poor outcomes in patients with COVID-19 as compared to a CCI score of 0.25 Each incremental point in the CCI score raised the mortality risk by 16%. Thus, a higher mean CCI score was significantly associated with both mortality and disease severity.25 These and similar findings were used by Nuevo-Ortega et al., who proposed a method for predicting the prognosis of patients with COVID-19 pneumonia.46 The method combines age-adjusted CCI and the pneumonia severity scale (CURB-65), with the addition of the measurement of arterial saturation with pulse oximetry as the only supplementary diagnostic tool.46
This study is the first research in Ukraine to evaluate the impact of the comorbidity burden, quantitatively assessed by applying CCI, on the severity of CAP caused by SARS-CoV-2. Our results demonstrated that patients with higher CCI scores had more severe CAP. This finding suggests that the CCI score can be used as an alternative to pneumonia-specific severity scores, not only to predict the risk of death. Moreover, our research can be useful for identifying patients who are at high risk of poor outcomes and may guide clinical decision-making.
Limitations
The present study has several limitations. First, its retrospective character makes it susceptible to knowledge bias. Second, some previous medical history was not classified according to the International Classification of Diseases 10th Revision (ICD-10) diagnosis codes, and some information about comorbidities might be missing. Third, the duration of COVID-19 illness prior to hospitalization was not considered in this study. Fourth, the sample size of the risk class IV group was limited. Additionally, we cannot rule out the hypothesis that the patients included in the study do not represent the entire cohort of patients with CAP caused by SARS-CoV-2 in Ukraine. However, our results reflect a heterogeneous real-world population representative of clinical practice.
Conclusions
The present study suggests that the comorbidity burden has a significant impact on the severity of CAP caused by SARS-CoV-2. The majority of patients in the pneumonia risk class II and risk class III groups had a low CCI score of 0–2, while the majority of patients in the risk class IV group had a high CCI score (≥3). Moreover, a direct correlation was established between the CCI score and the age of inpatients with CAP caused by SARS-CoV-2. The use of CCI to evaluate comorbid pathology in hospitalized patients with CAP caused by SARS-CoV-2 can assist the medical staff in developing timely preventive and therapeutic strategies, leading to improved patient prognosis.
Ethical approval and consent to participate
The study protocol was approved by the Ethics Committee at I. Horbachevsky Ternopil National Medical University, Ukraine (No. 73, 03.04.2023).
Data availability
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.