Abstract
This commentary on sleep medicine explores whether the potential relationship between sleep bruxism (SB), masticatory muscle pain (MMP) and sleep breathing disorders (SBDs) contributes to improving the management of co-occurring conditions.
The paper is divided into 2 sections: (1) reviewing the debate on SB nosology; and (2) based on the publications from the Martynowicz & Wieckiewicz research group, exploring the role of intermittent hypoxia as a putative mechanism endotype that may link such co-occurrence among individuals for whom characteristics are not yet clear.
Keywords: sleep bruxism, masticatory muscle pain, sleep breathing disorders, hypoxia, nosology
Disentangling the nosology of sleep bruxism is essential before associating it with other conditions and investigating mechanisms
According to the consensus from a group of clinical scientists presented in a work-in-progress report, sleep bruxism (SB) is defined as “masticatory muscle activity during sleep that is characterized as rhythmic (phasic) or non-rhythmic (tonic), and is not a movement disorder or a sleep disorder in otherwise healthy individuals”.1 Sleep bruxism is currently considered an oro-motor behavior, with central interaction between the cerebral and cardiorespiratory autonomic systems. The specificity of the genetic contribution remains to be confirmed.2
Since it has become accepted that SB is not a parafunction or a disease, it remains a matter of debate whether it fits into the classical scheme of a disorder in otherwise healthy individuals.1, 2, 3 Difficulty with clarifying whether SB is a behavior or a disorder is evident in a series of landmark commentaries; it is due to the gray area between a behavior without consequences and the point at which it becomes harmful.4 Therefore, standardization is required so that firm, evidence-based conclusions could be generalized to clinical practice.5 This is further corroborated by a recent theoretical analysis that does not support classifying SB as a disorder.6, 7 The American Academy of Sleep Medicine (AASM) in the International Classification of Sleep Disorders – Third Edition, Text Revision (ICSD-3-TR) insists on using the word “related”, since many sleep-related movement conditions do not entirely fit into the nosology of sleep disorders.6 In a theoretical reappraisal of the ICSD-3 listing and definitions of disorders, it becomes even more clear that SB does not fit into the classical harmful dysfunction analysis (HAD) framework due to the lack of solid evidence.7 Based on the above citations, it is evident that much more work is needed to identify rigid criteria and biomarkers for SB.
It remains perilous to directly and equally combine SB with MMP or SBDs. Although multiple associations have been found when analyzing population samples, not many are solid enough to guide our practice. For example, the phenotyping of obstructive sleep apnea (OSA) and the search for more definitive markers are ongoing. Therefore, the causal relationship between SB, MMP and SBDs should be considered on a personalized basis, as generalization is premature in the absence of unequivocal evidence.8
The resolution to this controversy may lie in pathophysiological, biochemical and molecular mechanisms, as well as genetic predispositions, leading to a cause-and-effect relationship or coexistence in some individuals.
Intermittent hypoxia as a common putative endotype of masticatory muscle pain in co-occurring sleep bruxism and sleep breathing disorders
Research by Wieckiewicz et al.9 and Smardz et al.,10 utilizing the video-polysomnographic evaluation of SB, did not demonstrate a direct relationship between temporomandibular disorders (TMDs) and TMD-related pain intensity in SB patients, with the distribution of TMDs among sleep bruxers and non-bruxers being similar. Therefore, it cannot be unambiguously assumed that a significant number of SB episodes alone is a risk factor for a higher prevalence of TMDs and TMD-related pain, including MMP. There are probably other factors related to sleep that could potentially affect this prevalence.
A recent study by Martynowicz et al. revealed a correlation between the asymmetry of temporal muscle pain and the presence of rhythmic masticatory muscle activity (RMMA) clusters in SB, with no such results being observed for masseter muscle pain.11 An RMMA cluster was defined as a group of at least 3 RMMA events (the phasic or mixed form) with 3 or more burst increases lasting 0.5–2 s and an interval between the cluster events no longer than 50 s.11 Furthermore, Seweryn et al. demonstrated a statistically significant association between a poor sleep quality and higher levels of temporal muscle pain.12 This association was not observed for masseter muscle pain.12 Therefore, it is not only the number of bruxism episodes, but also their duration, severity, structure, and other pathomechanisms that potentially influence MMP. Moreover, it can be assumed that temporal muscle pain is potentially more dependent on SB and sleep quality than masseter muscle pain. These hypotheses necessitate further reliable research on large groups, using video polysomnography (VPSG) and the objective assessment of MMP intensity.
In a study on a group of generally healthy individuals, Suzuki et al. found that oxygen saturation (SpO2) was slightly but significantly lower than at baseline (maximum: −0.6%) at 4–6 s before the RMMA onset; in contrast, SpO2 was markedly higher at 6–18 s after the RMMA onset (0.9%).13 The end-tidal carbon dioxide (ETCO2) value before the RMMA onset did not differ from that at baseline, and it decreased 8–10 s after the RMMA onset (−1.7 mmHg). However, no changes in SpO2 or ETCO2 in relation to the RMMA onset reached a critical clinical threshold. The authors concluded that mild and brief oxygen fluctuations before the RMMA onset may reflect a physiological response that appears to have little influence on SB genesis.13
The relationship between SB and SBDs is different in patients with OSA. Martynowicz et al. found that individuals with mild and moderate OSA (apnea–hypopnea index (AHI) <30) had a higher bruxism episode index (BEI) as compared to those with severe OSA (AHI ≥ 30).14 A positive correlation between AHI and BEI was observed in the group with AHI < 30, highlighting the dependence of the relationship between OSA and SB on OSA severity. In patients with mild and moderate OSA, OSA was found to be correlated with SB. The study also demonstrated a positive linear correlation between phasic bruxism and the oxygen desaturation index (ODI), as well as between phasic bruxism and minimal SpO2, in individuals with AHI < 30, confirming the association between hypoxia and SB.
Smardz et al. found a significant relationship between tonic electromyographic pathways in SB episodes and SBDs, with tonic muscle contractions identified as the potential cause and effect of respiratory events.15 Qualitative analysis revealed a statistically significant correlation between increases both in AHI and ODI and an increase in tonic electromyographic pathways.15
Concluding remarks
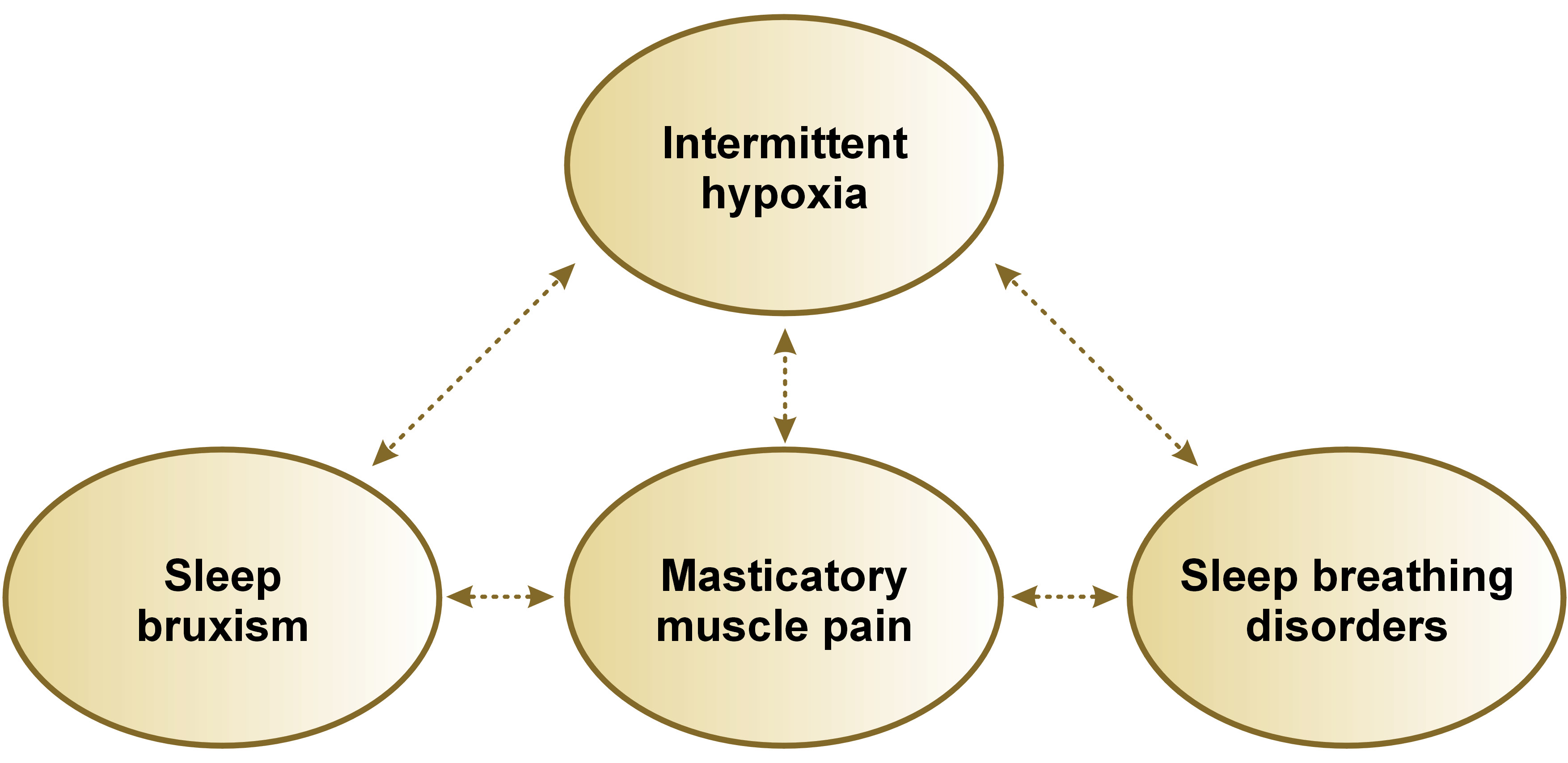
Based on the results presented above, it can be concluded that repeated episodes of moderate or severe intermittent hypoxia may be the potential factor connecting SB, MMP and SBDs (Figure 1). However, the authors emphasize that this is likely one of many factors, and a multifactorial etiology is more probable. Therefore, high-level evidence studies are needed to confirm or reject this hypothesis.
Clinicians should be aware that repeated episodes of moderate or severe intermittent hypoxia are harmful to the cardiovascular system,16 causing sympathetic hyperexcitation and contributing to an increase in the generation of reactive oxygen species (ROS), systemic inflammation, metabolic dysregulation, and endothelial dysfunction,17 and potentially leading to faster growth of cancerous tumors.18, 19, 20 Further investigation is required to determine the possible link or causal role of these factors in the interaction of SB with MMP and SBDs with MMP, and to provide innovation in the management of such putatively deleterious co-occurring conditions.