Abstract
Background. Platelet-rich fibrin (PRF) is widely used in periodontics for its wound healing potential. Two major variations of PRF are the original leukocyte- and platelet-rich fibrin (L-PRF) and the modified low-speed advanced PRF (A-PRF).
Objectives. The aim of the present study was to evaluate and compare the conventional L-PRF protocol and the low-speed A-PRF protocol in terms of angiogenic potential of PRF, using an in vivo chorioallantoic membrane (CAM) assay.
Material and methods. Fifteen fertile Giriraja eggs were procured and after a 3-day incubation period, randomly allotted into 3 groups: control; L-PRF; and A-PRF. A total of 20 mL of blood was collected from systemically healthy male volunteers aged 18–24 years, using a standard protocol. The PRF samples were inoculated on the CAM of the eggs. On the 10th day, the eggs were reopened and photographed. The parameters assessed were the number, length, size, and density of blood vessels, as well as the number of junctions formed. The photographs were analyzed using the ImageJ and ProgRes® CapturePro software.
Results. Seven days after inoculation, both the A-PRF and L-PRF groups exhibited significantly better results than the control group in terms of the number (59.20 ±6.61 vs. 48.80 ±5.07 vs. 19.20 ±6.98), length (25,000 ±1,813.10 μm vs. 17,000 ±282.90 μm vs. 8,000 ±184.49 μm), size (230,000 ±15,054.00 μm2 vs. 200,000 ±8,295.27 μm2 vs. 150,000 ±4,105.16 μm2), and density (central: 9,100 ±296.78 vs. 5,370 ±272.42 vs. 1,420 ±564.36; peripheral: 9,094 ±400.14 vs. 3,370 ±479.39 vs. 5,420 ±746.73) of blood vessels, as well as the number of junctions formed (52 ±3.81 vs. 41 ±1.58 vs. 33 ±4.64), respectively.
Conclusions. The angiogenic potential was increased by the exposure to both L-PRF and A-PRF. However, A-PRF demonstrated statistically significant benefits in terms of the number, length, size, and density of blood vessels, as well as the number of junctions formed in comparison with the control and L-PRF groups.
Keywords: angiogenesis, wound healing, platelet-rich fibrin, chorioallantoic membrane assay
Introduction
Chronic periodontitis inevitably leads to tissue loss or damage, making the restoration of tissues a challenging task due to the difficulty in repairing and regenerating the periodontium. Therefore, seamless wound healing is a crucial factor in determining the success of periodontal therapy.1
The literature describes various techniques regarding the stimulation of the wound healing process, either naturally or artificially.2 The artificial pathway involves the use of chemical agents, such as hormones (e.g., human growth hormones, insulin and testosterone), or physical means, such as photobiomodulation and heat therapy. The natural pathway is accelerated by using autologous materials, such as platelet concentrates, or herbal extracts, like green tea or the kiwi extract. The selection of the technique depends on the size and location of the tissue to be regenerated, the clinician’s expertise, patient preference, affordability, and the feasibility of the required surgical procedure.3
Regenerative periodontics and tissue engineering aim to restore both the structure and function of the damaged tissues. These emerging approaches employ specific biocompatible and bioactive composites or natural scaffolds, into which cells or bioactive molecules are incorporated to construct a dynamic environment for wound healing within the damaged tissues. Several recent studies have focused on autologous platelet concentrate derivatives, which may delay complications and boost tissue regeneration. Platelet derivatives were first introduced by Choukroun et al. in 2001,4 and several modifications of the original protocol are currently in use. Platelet-rich fibrin (PRF) is a second-generation platelet concentrate obtained by centrifuging the patient’s blood without any external additives, such as anticoagulants. Currently, low-speed advanced PRF (A-PRF) and conventional leukocyte- and platelet-rich fibrin (L-PRF) are popular for various dental applications, including the treatment of necrotic tissue, like pulp and interdental papillae, through pulp revascularization and papilla regeneration, in ridge augmentation, orofacial reconstruction, and the repair of an oro-antral fistula, and intrabony or furcation defects. They can also be used as a bandage over soft tissue donor sites and for recession coverage.3, 4, 5, 6
The major rationale for the use of PRF is that angiogenesis is the cornerstone of all biochemical processes in our body. A few studies have quantified the level of vascular endothelial growth factor (VEGF), but none has directly compared the angiogenic potential of different types of PRF or various PRF protocols. Since the angiogenic properties of the biomaterial contribute immensely to its wound healing potential, the present study may help clinicians use the available resources wisely and judiciously, thereby reducing the cost of additional regenerative materials.7
The study aimed to evaluate and compare the conventional L-PRF protocol and the low-speed A-PRF protocol in terms of angiogenic potential of PRF, using an in vivo chorioallantoic membrane (CAM) assay.
Material and methods
This controlled in vitro study was approved by the Institutional Review Board at Bapuji Dental College and Hospital, Davanagere, India (approval No. BDC/509/2019-20), and was conducted in accordance with the Declaration of Helsinki of 1975, as revised in 2000. Blood samples were collected from systemically healthy male volunteers aged 18–24 years. Volunteers were excluded from participating in the study if they were heavy smokers or on drug therapy that might affect the outcomes of the study. Three groups were created: control; L-PRF; and A-PRF. Two different types of PRF were prepared, i.e., L-PRF was prepared using the standard protocol of 2,700 rpm for 12 min,8 while A-PRF was obtained by centrifuging blood at 1,500 rpm for 14 min.9
The samples were inoculated on the CAM of fertile Giriraja eggs. The assay was conducted randomly in pentaplicate, and the eggs were reopened on the 10th day of incubation to record the results. Photographs were taken on the 3rd and 10th day, and the images were analyzed using the ImageJ 1.53k software (https://imagej.net/ij) bundled with the Java analysis software, v. 1.8.0_172,10 and the ProgRes® CapturePro software, v. 2.8.8 (Jenoptik, Jena, Germany).11 The parameters assessed included the number, length, size, and density of blood vessels, as well as the number of junctions of blood vessels.
Platelet-rich fibrin preparation
Approximately 20 mL of venous blood was collected from each volunteer, using a needle and a sterile plastic vacutainer tube for the preparation of PRF. The blood samples were transferred into 2 sterile 10-milliliter glass tubes without anticoagulation, one for L-PRF and the other for A-PRF. The tubes were immediately centrifuged (PRF Duo Quattro; Ostralos Ltd, Auckland, New Zealand), using a standard protocol.
Platelet-rich fibrin was prepared by a single investigator according to the protocol described by Dohan et al. in 20048 for L-PRF and Ghanaati et al.9 for A-PRF. Leukocyte- and platelet-rich fibrin was obtained by centrifuging 10 mL of blood in a red-capped L-PRF tube at 2,700 rpm for 12 min, while A-PRF was prepared by centrifuging 10 mL of blood in a red-capped A-PRF tube at 1,500 rpm for 14 min. In both cases, 3 layers were obtained: platelet-poor plasma (PPP); a PRF clot; and a red blood cell (RBC) layer. The fibrin clot was easily separated from RBCs at the bottom. For both types of PRF, 5 samples were prepared.
Chorioallantoic membrane assay
The CAM assay was conducted at the Government Veterinary Hospital in Bengaluru, India.
The study was carried out according to the standard protocol12 and involved the following steps:
1. Obtaining fertile eggs: 15 fertile Giriraja chicken eggs, weighing approx. 58 g each, incubated for 72 h at 37°C and 70–80% humidity, were obtained from the egg hatchery at the Department of Poultry, Government Veterinary Hospital, Bengaluru, India;
2. Incubation: The eggs were incubated in the Multiquip E2 incubator (Multiquip, Sydney, Australia) at 37°C and 60% humidity. The egg tray was automatically tilted by 45° every 30 min to simulate the natural process;
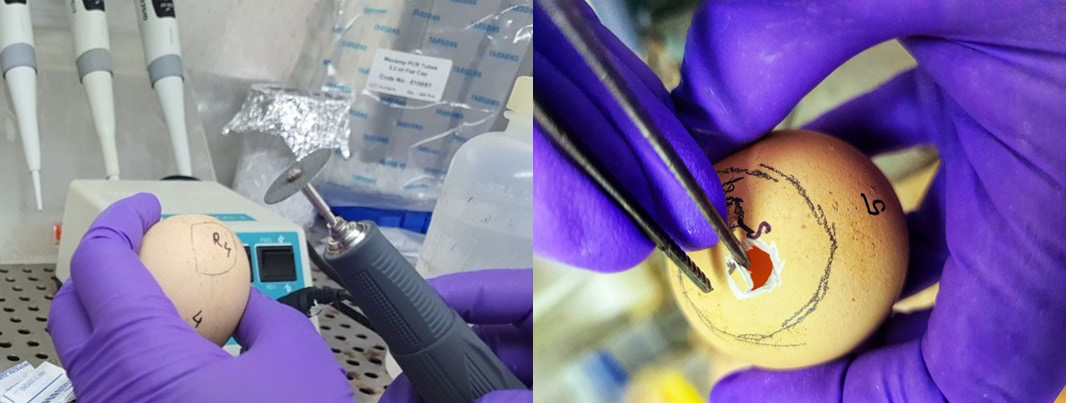
3. Disinfection: The eggshells were disinfected with a 70% ethanol solution for 2–3 min (Figure 1);
4. Candling: Candling of the embryos was performed to confirm egg fertility and determine the position of the air sac (Figure 2), thus establishing the optimal position for the placement of the biomaterial on CAM. A pencil was used to mark the opening area;
5. Opening: On day 3 of chick embryo development, a small opening was made in the shell under aseptic conditions, using a wheel bur and a blunt tweezer (Figure 3);
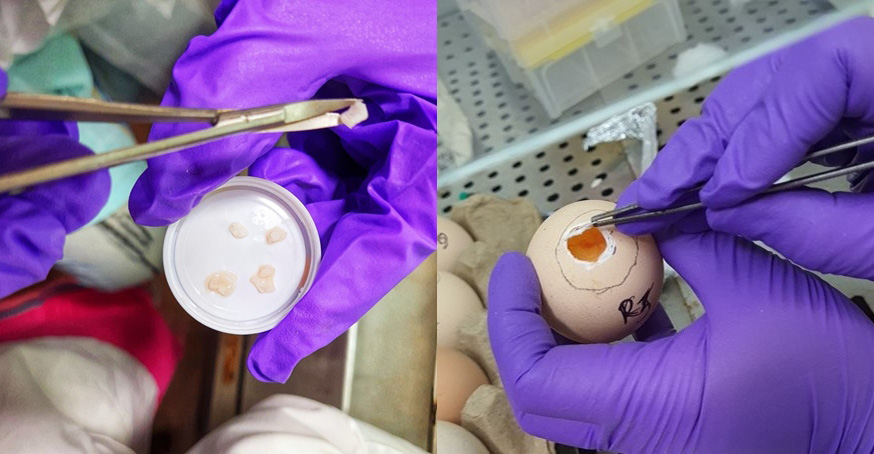
6. Inoculation: The eggs were divided into 3 groups: control; L-PRF; and A-PRF. Both L-PRF and A-PRF were cut into uniform fragments measuring 1 mm × 2 mm, and were carefully inoculated on the CAM of the eggs over the blunt end of the egg, where the opening was made (Figure 4);
7. Sealing: The opening was resealed with paraffin wax and the eggs were returned to a mini-incubator for the next 7 days (Figure 5);
8. Reopening: After disinfecting the eggs with a 70% ethanol solution, they were dewaxed manually using a hot instrument. The images were taken after the contents of the eggs were transferred to a Petri dish together with CAM (Figure 6).
The obtained images were analyzed to determine the effects of the biomaterial on the angiogenesis process in the CAM of the developing chick embryo.
Image analysis
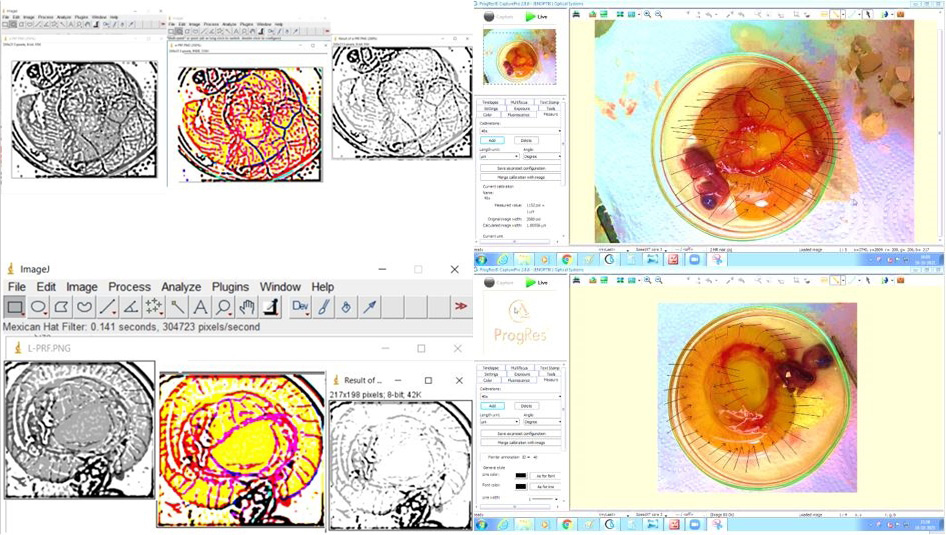
After applying the Mexican Hat Filter, conversion to 8-bit and the measurement of the vessel area, the density of the vascular network was quantified using the ImageJ software.13
Morphometric analysis was carried out using the ProgRes CapturePro software. For vascular morphometric analysis, the images were captured using a 64MP wide-angle primary digital camera (Samsung Galaxy F62; Samsung Electronics Co., Ltd., Suwon, South Korea), set at F1.8, 1/50 s, 5.23 mm, ISO 200, with auto white balance and no flash. The measurements were made using the ProgRes CapturePro software. To ensure accuracy, we calibrated the magnification, using a stage micrometer before measurement. The images were saved and recalled on the monitor. All measurements were taken using the software measuring tools.
Stage readings were reviewed for reassessment. The number of vessels was measured in each group, and black arrows were used to mark recognizable vasculature. The length of the total vasculature was measured in micrometers, the size of the vessels was recorded in micrometers squared and the number of junctions of blood vessels was calculated using the ProgRes CapturePro software by counting the total number of branch points. The density of blood vessels was determined using the ImageJ software (v. 1.38) by measuring the amount of red pixels per area unit (Figure 7).
The analysis was conducted by 2 independent observers to minimize subjectivity.
Statistical analysis
The obtained results were tabulated and subjected to statistical analysis. The mean and standard deviation (M ±SD) values were calculated for all parameters. The one-way analysis of variance (ANOVA) was used to compare the results obtained for the replicas within the groups. The Bonferroni test was used to analyze differences between the groups for multiple comparisons of each parameter. A p-value <0.05 was considered statistically significant.
Results
All images were analyzed using 2 software programs – ImageJ and ProgRes CapturePro. No complications, such as embryo death, contamination or inclusion bodies, were observed during the study period. Both the A-PRF and L-PRF groups showed a significant increase in the number and density of blood vessels (p < 0.01) as compared to the control group (Table 1). In the peripheral zone, a small but dense vasculature spread was noted in the L-PRF group. In contrast, the A-PRF group exhibited more apparent and larger vessels, which were densely distributed throughout the tissues. The branched networks in the A-PRF group were also large in size and showed a high density (Table 1). Both the A-PRF and L-PRF groups showed a significant increase in the length of blood vessels as compared to the control group (p < 0.001). The A-PRF group presented a greater length of blood vessels than the L-PRF group (Table 1). Additionally, the A-PRF group had a significantly greater size of blood vessels than the L-PRF and control groups (Table 1). The A-PRF group also demonstrated an increased number of junctions as compared to the control and L-PRF groups (Table 1).
Discussion
Healing involves the restoration of both quantity and quality of healthy tissues through regeneration and repair. Angiogenesis is a fundamental concept in all physiological as well as pathological events in a biological system. Disturbances in angiogenesis may result in cancer, rheumatoid arthritis, psoriasis, retinopathies, and obesity, when it is increased, and in ulcers, chronic wounds, stroke, and even coronary artery disease, when it is decreased.14 Our study focused on finding an ideal biomaterial to promote wound healing, with good predictability, reduced surgical time and minimal morbidity.15
Autologous platelet concentrates are an ideal option, as they contain concentrated autologous growth factors that stimulate stem cells, attract them to the injured site, stimulate angiogenesis, enhance immunity, act as a scaffold, and encourage wound healing. The use of PRF has been frequently reported in the literature with regard to regeneration. Platelet-rich fibrin offers several benefits, including antibacterial efficacy, root conditioning properties and recession coverage. It is used for the treatment of the chronic ulcers caused by diabetes or burns. Platelet-rich fibrin also promotes osteogenesis through its osteoconductive efficacy, although its osteoinductive properties have not yet been established.16
Leukocyte- and platelet-rich fibrin is a second-generation platelet concentrate, introduced by Dohan et al. in 2004.8 In an in vitro study, the use of L-PRF resulted in a very strong stimulation and proliferation of endothelial cells, pericytes, fibroblasts, and pre-keratinocytes for more than 28 days.17 In another study, it was found that the growth factor release profile of L-PRF was up to 7 days.18 For such reasons, L-PRF is widely used in the treatment of periodontal defects, as well as in systemic applications for diabetic foot ulcers, venous ulcers and others.17, 18
In 2014, Ghanaati et al. developed a new protocol concept for a low centrifugation speed.9 It was based on the fact that a low speed helps in the even distribution of platelets, increases their amount and results in greater leukocyte entrapment throughout the fibrin clot. The protocol was named A-PRF.19, 20 Histological and biochemical studies revealed that A-PRF was more porous, heavily packed with monocytes and platelets, and uniformly saturated with growth factors. Moreover, it was shown that this type of PRF had a higher growth factor release profile for up to 10 days as compared to L-PRF.21
Recent research and clinical trials have concluded that the superior healing properties of L-PRF and A-PRF are related to their chemoattractive, angiogenic, osteogenic, anti-inflammatory, anti-microbial, pain-inhibitory, and wound healing characteristics.22 Various studies have shown improved bone regeneration and soft tissue regeneration when using these biomaterials, with or without bone grafts.23 However, angiogenesis is a complex procedure that involves a sequential interplay of various cells, growth factors and environmental factors.24 The aim of this study was to evaluate the angiogenic efficacy of conventional L-PRF vs. low-speed A-PRF, as there is a lack of literature directly comparing the in vivo angiogenic potential of these 2 commonly used PRF protocols.
The chick CAM assay is one of the oldest and most widely used methods for studying angiogenesis in vivo. It was developed by Folkman in 1974,25 and takes advantage of the fact that CAMs are present in the fertile eggs of all avian species, they are immunodeficient and contain numerous blood vessels. This structure rapidly expands, generating a rich vascular network that enables the examination of tissue grafts, tumor growth, wound healing, drug delivery, and angiogenic and anti-angiogenic molecules, as well as toxicological analysis. These characteristics are ideal for in vivo assays. The method is reproducible, fast, suits large-scale screening, and allows the simple visualization of new vascularization under a microscope. Also, this assay is one of ethically acceptable methods of investigating angiogenesis in vivo.26, 27
We selected the CAM assay as the model to study angiogenesis due to its abovementioned benefits. The eggs used in the study were procured from the same hatchery and were at the same stage of incubation to minimize bias. In a recent in vivo-in vitro randomized controlled trial on the effect of adding PRF to 3 different types of porcine collagen membranes (mucoderm®, collprotect® and Jason®), the CAM assay was selected as the in vivo model to study angiogenesis.27
Miron et al. conducted a study on male patients aged 20–40 years to prepare PRF from blood samples and eliminate bias based on the relationship between age, gender and healing potential.28 Smoking and nicotine have negative effects on epithelial cell proliferation and connective tissue interaction, which are essential steps in wound healing. Therefore, we excluded chronic smokers from our study protocol.29 Additionally, systemically compromised patients, such as those with bleeding disorders, diabetes mellitus, or patients on drug therapy that might affect the outcomes of the study were excluded.
All parameters, including the number of blood vessels formed, the total vasculature length, the blood vessel size, the vascular network density, and the number of junctions of blood vessels, were analyzed using 2 software programs, ImageJ11, 12 and ProgRes CapturePro,30after processing the images taken at the end of the 10th day. Blood vessel count was performed both quantitatively and qualitatively.
During the qualitative analysis, we observed strong neovascularization in the A-PRF group and moderate neovascularization in the L-PRF group as compared to the control group. Both the L-PRF and A-PRF groups showed significantly higher blood vessel formation, greater density, increased length, and larger size of blood vessels as compared to the control group. A recent in vivo-in vitro study compared the angiogenic efficacy of PPP, platelet-rich plasma (PRP) and PRF.31 The concentrations of angiogenic factors and their bioactivity were determined, and the results showed that in the PRP and PRF preparations, both VEGF and platelet-derived growth factor BB (PDGF-BB) were significantly more concentrated than in PPP, whereas PRF was the most effective for wound closure. In the CAM assay, the PRF membranes were the most effective for neovascularization.31 The results of previous studies31, 32 are in agreement with our outcomes, as L-PRF and A-PRF demonstrated significant neoangiogenesis both quantitatively and qualitatively. Another study was conducted to evaluate the angiogenic potential of L-PRF using in vitro and in vivo assays.33 The in vitro assay utilized an antibody array to determine the growth factors released by L-PRF. High levels of CXC chemokine receptor 2 (CXCR-2) ligands and epidermal growth factor (EGF) were reported. The in vivo study was conducted using a CAM assay. It was found that L-PRF induced in vitro the key steps of the angiogenic process, including endothelial cell proliferation, migration and tube formation, thus accelerating angiogenesis.33 However, no such study has been conducted for A-PRF.
Advanced PRF showed a significantly higher blood vessel density, centrally and peripherally, longer blood vessels, more junctions, and larger blood vessels than both the control group and the L-PRF group (p < 0.05). In a recent study, the release of growth factors such as PDGF-AA, transforming growth factor beta 1 (TGF-β1), VEGF, EGF, and insulin-like growth factor 1 (IGF-1), was assessed; it was found that the release of VEGF for L-PRF and A-PRF on the 1st day was 106 pg/mL and 150 pg/mL, and on the 10th day, it was 175 pg/mL and 210 pg/mL, respectively.32 The study reported that A-PRF released more growth factors than L-PRF, indicating that the low-speed concept is more effective.32 The low-speed concept leads to a more even distribution of platelets and growth factors throughout the clot matrix, unlike the conventional protocol, where most growth factors concentrate just above the RBC layer.33 Therefore, it can be concluded that reducing the centrifugation speed significantly enhances angiogenesis. Our study also confirms the superior performance of A-PRF, which can be attributed to an increased diffusion and dispersion of growth factors from A-PRF as compared to L-PRF.
Overall, the results of our study demonstrate that both A-PRF and L-PRF have strong angiogenic properties. The limitations of the present study include the absence of histological and immunological evaluations.34 However, the present study provides new insights with regard to the future of angiology and regenerative periodontics.
Conclusions
Exposure to both L-PRF and A-PRF increased the angiogenic potential. Advanced PRF demonstrated a statistically significant enhancement in the number, length, size, and density of blood vessels, as well as in the number of junctions of blood vessels. Further in vivo and in vitro studies using different models of angiogenesis are recommended to determine the suitability of these materials as ideal wound healing agents.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board at Bapuji Dental College and Hospital, Davanagere, India (approval No. BDC/509/2019-20).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.