Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease with various oral manifestations, including ulceration, white keratotic plaques, oral discoid lupus erythematosus, oral lichen planus (OLP)-like lesions, non-specific erythema, purpura, petechiae, and cheilitis, which resemble lesions of other systemic diseases. Recognizing the oral manifestation of SLE is essential for comprehensive patient management. This study reports 4 cases of SLE with various oral lesions, underlying conditions and diagnostic methods.
In September 2019, 2 adult SLE patients and 2 juvenile SLE patients were consulted at the Oral Medicine Clinic. The assessment of systemic diseases was conducted by the Internal Medicine and Pediatrics resident, whereas the Oral Medicine resident performed the intraoral examinations. The medical history, clinical findings and laboratory results were analyzed to establish the diagnosis.
The first patient was a 38-year-old female presenting with multiple white keratotic plaques throughout the mucosa, an OLP-like lesion on the right buccal mucosa, petechiae on the hard palate, and petechiae and purpura on the upper and lower extremities. The second case was a 24-year-old female with a malar rash and multiple ulcerations on the vermilion zone, an OLP-like lesion on the left buccal mucosa, and a palatal ulcer. The third and fourth cases were 16-year-old females with a prominent butterfly rash. The patients presented with acute pseudomembranous candidiasis, an aphthous-like ulcer and keratotic plaques. They received antimicrobial therapy for the intraoral lesions and showed promising results.
The oral lesions in adult- and juvenile-onset SLE patients varied depending on the disease severity and treatment received.
Keywords: systemic lupus erythematosus, oral lesions, adult SLE, juvenile SLE, oral ulcer
Introduction
Lupus erythematosus (LE), commonly referred to as systemic lupus erythematosus (SLE), is an autoimmune disease that can affect multiple organs and present with various clinical manifestations.1, 2 The incidence of SLE has increased in recent years, with reported rates of 2–8 cases per 100,000 individuals in Europe, South America and North America, 51 cases per 100,000 individuals in the USA,1 and 30–50 cases per 100,000 individuals in Asia, where the incidence varied from 0.9/100,000 to 3.1% per year. The epidemiological data on SLE differs between Asian countries and is difficult to generalize. However, there are similarities in the clinical presentation of the disease.3 In 2010, 291 SLE patients were registered at the Rheumatology Clinic of Dr. Hasan Sadikin Central General Hospital (Bandung, Indonesia), accounting for 10.5% of all patients registered at the Rheumatology Clinic.1
In 1997, the American College of Rheumatology (ACR) issued a set of diagnostic criteria for SLE, which were revised in 2012 by the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) to be more sensitive but less specific.4 These criteria are used to diagnose SLE in children and adults. Several studies reported that oral lesions in SLE varied from 9% to 45% and from 3% to 20% in localized cutaneous disease.5, 6, 7 In 2019, the European League Against Rheumatism (EULAR) and the ACR approved new criteria for SLE, which require a positive antinuclear antibody (ANA) test. The new criteria have 96.1% sensitivity and 93.4% specificity, compared with 82.8% sensitivity and 93.4% specificity of the ACR 1997 criteria and 96.7% sensitivity and 83.7% specificity of the SLICC 2012 criteria.8
Many terms have been used to describe the LE oral lesion, including oral discoid lesion, chronic plaque, lupus cheilitis, acute ulcer, oral ulcer, red ulcer, ulcerative plaques, pebbly red area, honeycomb lesion, keratotic lesion, white keratotic plaques, purpuric lesion, and diffuse palatal petechial erythema.9, 10, 11, 12, 13 A study in the Hungarian population reported that lupus nephritis, hematological disorders, photosensitivity, butterfly rash, and mucosal ulceration are more common in children than in adults, whereas neurological symptoms and polyarthritis occur more frequently in adults.14 The present article describes 4 clinical cases of various oral lesions identified in patients with SLE, along with their diagnosis and management.
Material and methods
This article is a case series study with a prospective design conducted in a single center using consecutive sampling. The patients were admitted to Dr. Hasan Sadikin Central General Hospital (Bandung, Indonesia), a government-run academic and community hospital. An Internal Medicine and Pediatrics resident, supervised by their consultant, performed the examinations of systemic conditions and laboratory assessments needed to support the diagnosis. At the same time, an Oral Medicine resident conducted intraoral examinations under the supervision of their consultant. The final diagnosis was based on patient complaints, clinical (extraoral and intraoral) observations and laboratory findings. The clinicians provided medication for systemic diseases, whereas intraoral lesions were treated by oral medicine specialists. Patient improvement was followed up for 3–4 weeks and any changes in medication type or dosage were reported. The results of the examinations, diagnosis, treatment, and oral lesion progress were documented in the patient’s medical records. The patients provided consent to document the intraoral lesions for further evaluation.
Results
The case series involved 4 female patients admitted to Dr. Hasan Sadikin Central General Hospital between September 2019 and December 2019. All patients were of reproductive age. Table 1 describes the clinical characteristics of each patient, with a butterfly (malar) rash and extraoral pale conjunctiva being the most common. Intraoral manifestations ranged from aphthous-like ulcers, keratotic plaques and oral lichen planus (OLP)-like lesions to central palatal erythema and acute pseudomembranous candidiasis. All patients were diagnosed with SLE according to the SLICC criteria, which are summarized in Table 2.
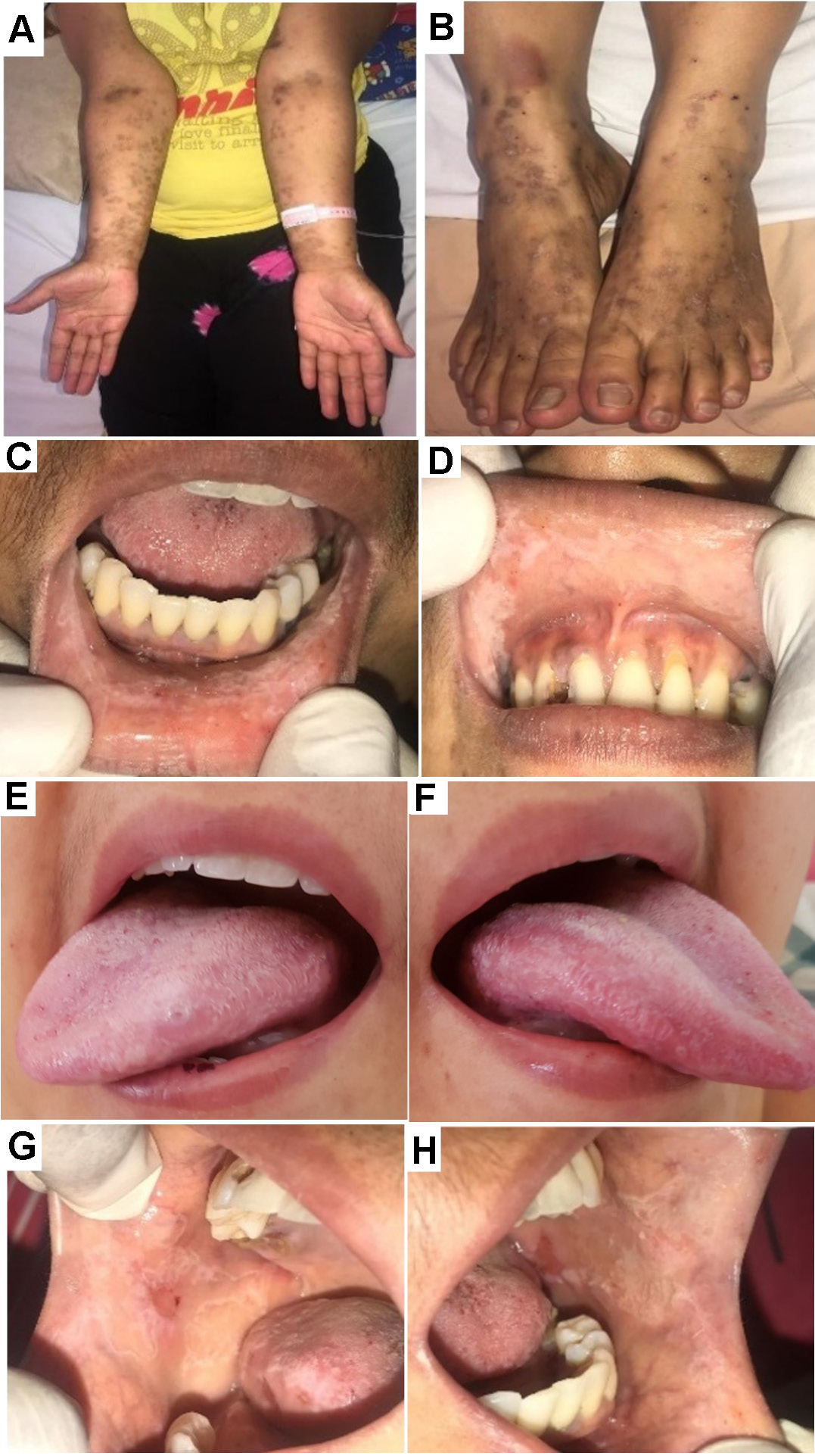
The first patient presented with secondary Evans syndrome, and the diagnosis of SLE was confirmed following intraoral examination and ANA testing, which indicates that intraoral findings may play a role in establishing a definitive diagnosis. Extraoral findings included petechiae and purpura on the upper and lower extremities, and pale conjunctiva (Figure 1, Figure ). Intraoral lesions included keratotic plaques on the upper and lower labial mucosa and the left and right lateral border of the tongue that could not be scraped (), unpainful lesions on the right buccal mucosa surrounded by whitish reticular plaque (OLP-like lesions) (Figure 1), whereas the left buccal mucosa showed no striae (Table 1). Multiple petechiae were also observed on the hard palate. The patient received the treatment listed in Table 3, was discharged 5 days later, and continued as an outpatient at a hospital closer to her hometown.
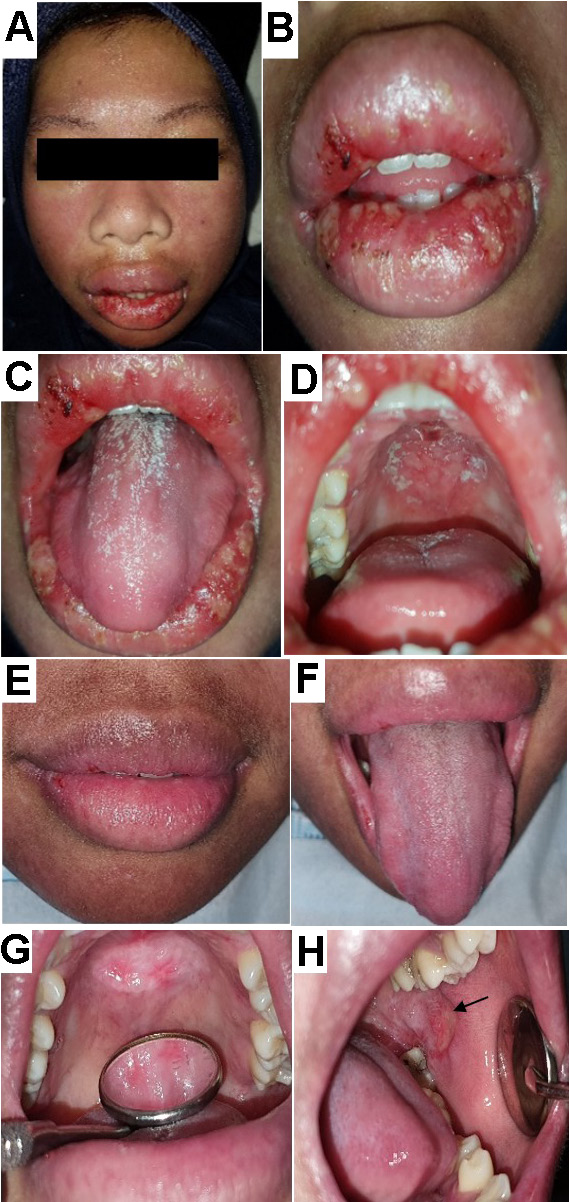
The second patient was diagnosed with SLE in April 2009 at another hospital, and was undergoing SLE treatment. She complained of swelling and ulceration of the lips. A few months earlier, she had similar symptoms after taking an antibiotic, which was confirmed to be an allergic reaction. The patient stated that the current swelling was not related to any medication. Extraoral findings revealed pale conjunctiva, a butterfly rash (Figure 2) and swelling of the lips associated with multiple minor ulcerations on the lower labial mucosa (Figure 2). Intraoral examination showed multiple ulcerations on the upper and labial mucosa as well as the left and right buccal mucosa. Additionally, acute pseudomembranous candidiasis (Figure 2) and a central erythematous lesion on the hard palate intermixed with whitish pseudomembranous plaques (Figure 2) were observed. The differential diagnosis consisted of herpes-associated erythema multiforme and drug-induced erythema multiforme. Herpes simplex virus-1 (HSV-1) and immunoglobulin E (IgE) serology were performed to rule out any possibility of hypersensitivity reaction and HSV involvement. The results for IgE were within the normal range and serology for HSV-1 was non-reactive. Therefore, intraoral HSV infection was excluded. Table 3 summarizes the patient’s medications.
One week later, the lip swelling, ulceration and oral candidiasis (Figure 2, Figure ) subsided. However, an unpainful central erythematous lesion on the hard palate (Figure 2) was revealed. Additionally, whitish radiant striae with central ulceration resembling OLP were observed on the left buccal mucosa (Figure 2). The patient was instructed to continue using the antimicrobial gel for the lesion on the palate and left buccal mucosa and petroleum jelly to reduce lip dryness. Selective grinding was performed on teeth 27 and 36 to minimize traumatic contact with the ulcer on the left buccal mucosa.
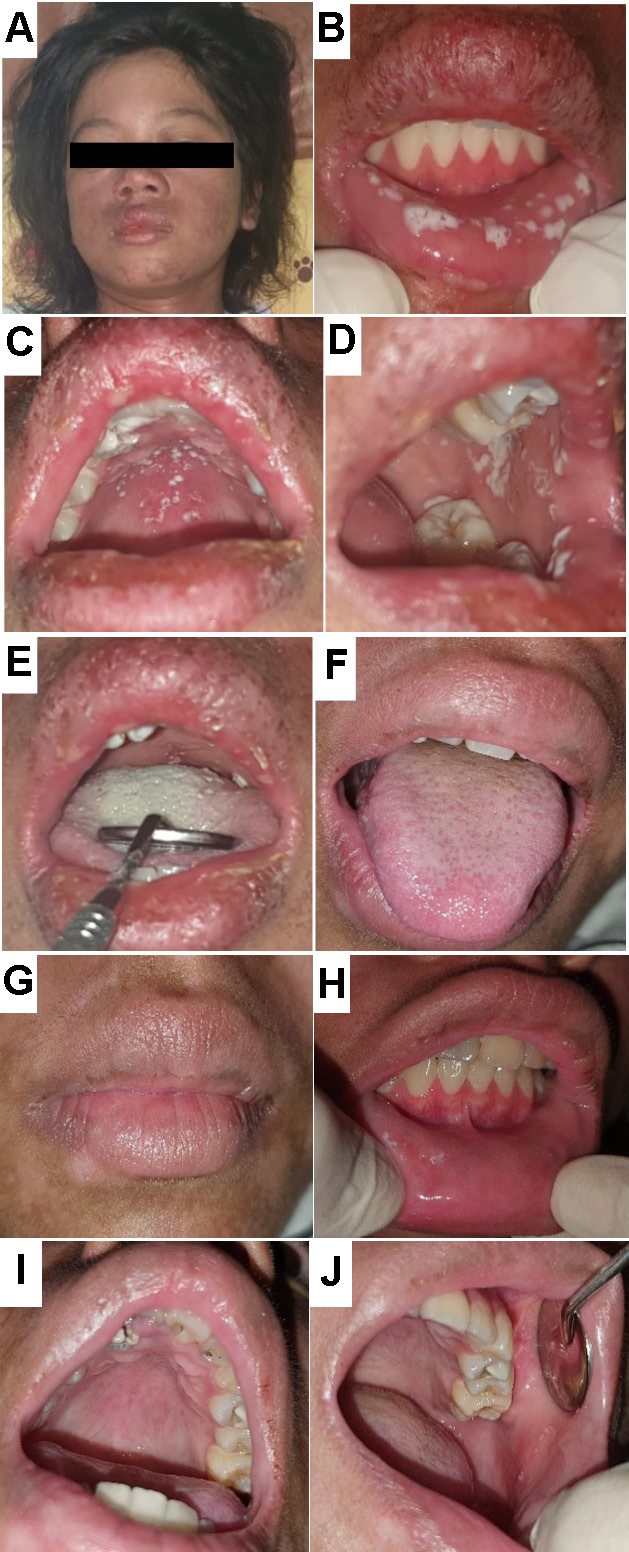
The third patient reported difficulty eating due to multiple ulcerations in the mouth. She had been treated for pulmonary tuberculosis for the past 2 months. However, a thoracic X-ray examination revealed that the pulmonary tuberculosis was not active and there was no cardiomegaly. Upon examination, we observed a prominent butterfly rash (Figure 3), pale conjunctiva, alopecia, dryness, and exfoliation of the vermilion. Intraoral findings revealed multiple scrapable white patches, leaving an erythematous base on the lower labial mucosa, hard palate and left buccal mucosa (). A thick coating was present on the dorsum of the tongue (Figure 3). Laboratory findings showed hemolytic anemia, elevated blood glucose, serum glutamic-oxaloacetic transaminase (SGOT), urea, and creatinine levels, and a reactive ANA test. Table 3 lists the patient’s medications.
Two weeks after treatment, the patient’s condition improved. The coated tongue showed marked improvement (Figure 3), the dryness of the vermilion decreased (Figure 3), and the oral candidiasis was reduced and almost completely resolved (). The patient was discharged and scheduled for regular control at the Pediatric Department, having followed the instructions for maintaining oral hygiene.
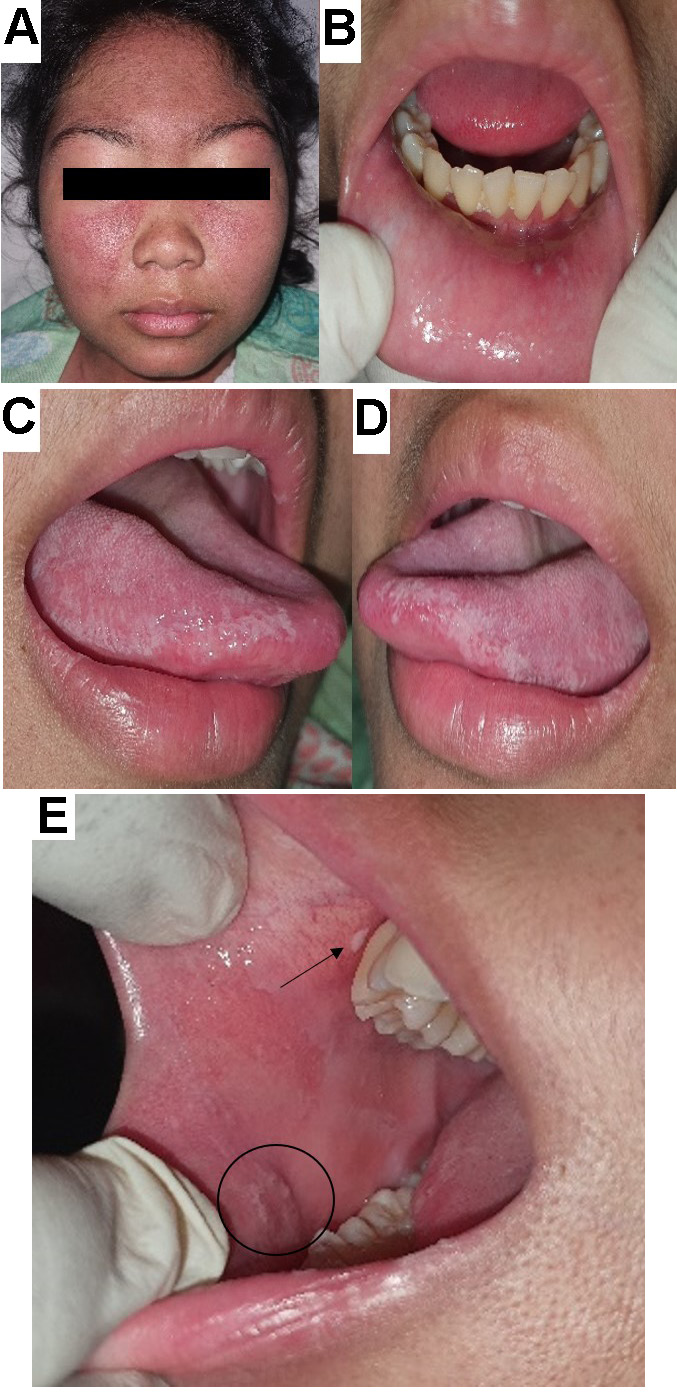
The fourth patient reported experiencing ulcerations in the mouth, pain while swallowing and pain inside the ear for 2 days before admission. The patient has been receiving treatment for SLE with skin and musculoskeletal involvement since March 2019. On examination, a butterfly rash was observed on her face (Figure 4). Intraorally, minor ulcerations surrounded by an erythematous area and a regular border were found on the lower labial mucosa opposite tooth 32 (Figure 4). Multiple faint, non-scrapable whitish plaques were observed at the anterior part of the left and posterior part of the right buccal mucosa (opposite teeth 44 and 46), and on the right and left lateral borders of the tongue (). The patient’s treatment is described in Table 3. Five days later, the ulceration on the lower labial mucosa disappeared, but the white keratotic plaques on the tongue and the faint whitish plaques on the right buccal mucosa persisted.
Discussion
Oral ulceration can be an indicator of various systemic diseases, such as LP, LE, benign mucous membrane pemphigoid, pemphigus vulgaris, Crohn’s disease, and Behçet’s syndrome. The ulcerations may present in various ways but maintain their characteristic features. In LE, oral lesions may present as oral discoid lesions, erythema, irregularly shaped ulcers, honeycomb plaques, raised keratotic plaques, purpura, petechiae, and cheilitis.15 These lesions may also be accompanied by other organ involvement or present as a solitary lesion. Therefore, it is crucial for clinicians to recognize the characteristic presentation of the disease to achieve an accurate diagnosis.
The first classifications of mucocutaneous SLE in the 1970s were divided into lupus-specific skin lesions (LE-specific) and non-specific skin lesions (LE-non-specific) and are used in both adult- (adult SLE) and juvenile-onset SLE (JSLE).16 The majority of the manifestations appear similarly in both groups. A study reported that the LE-specific butterfly rash and the generalized lupus rash were more frequent in JSLE than in adult SLE.17 In contrast, adult SLE patients were more likely than JSLE patients to present with subacute cutaneous lesions, discoid rash, generalized discoid LE (DLE), and LE panniculitis (profundus). In LE-non-specific cases, cutaneous vasculitis, oral and nasal ulcers, and bullous SLE were more common in JSLE patients, whereas photosensitivity, non-scaring alopecia, livedo reticularis, and Raynaud’s phenomenon were more frequent in adult SLE.17
The most common LE-specific lesion in JSLE and adult SLE is the butterfly rash. It presents as a symmetrical erythematous and edematous non-pruritic rash over the nasal bridge, typically sparing the nasolabial folds. This rash represents acute cutaneous lupus erythematosus (ACLE).18, 19, 20 In the present report, the butterfly rash was present in 3 out of 4 cases, with 2 patients reporting a recent diagnosis of SLE within the previous 2 weeks or 6 months.
Diagnosing SLE in the first patient was more challenging due to the absence of a butterfly rash. The patient presented with rashes on the upper and lower extremities, which are not specific to cutaneous LE. However, the presence of oral ulcers and laboratory findings of hemolytic anemia, leukopenia, thrombocytopenia, and a reactive ANA test helped establish the correct diagnosis. As stated in the literature, secondary Evans syndrome is linked to suspected underlying autoimmune diseases such as SLE, which causes hemolytic anemia, leukopenia and thrombocytopenia.21
In cases of adult SLE, oral ulcers may resemble reticular and erosive OLP, which typically present as a white lacy patch or Wickham’s striae and erythematous, ulcerated or erosive mucosa, frequently on the bilateral buccal mucosa and rarely on the palate. They may also resemble oral lichenoid reactions, which are usually associated with an adjacent metallic dental restoration. A biopsy of the lesion is required when the clinical presentation alone cannot establish a definitive diagnosis.22 However, in our study, a biopsy could not be performed in the first patient due to hemolytic anemia and thrombocytopenia, while the second patient refused to undergo the procedure. Moreover, the OLP-like lesions observed in our SLE patients were painless, unlike the erosive OLP, which may cause symptoms and interfere with patient activities.
Lip swelling may resemble angioedema, but it does not need to be accompanied by multiple ulcerations as observed in the second case. Erythema multiforme associated with HSV or induced by some drugs was also considered as a differential diagnosis due to the patient’s recent history of similar lip swelling associated with antibiotic treatment. However, we excluded the possibility of a hypersensitivity reaction due to the normal range of the serology test and the absence of causative antibiotic or drug consumption. Fortunately, the swelling subsided within a few days and the ulcerations, which may have been non-specific, healed.
The third patient did not have any SLE oral lesions, except for oral candidiasis. Multiple risk factors have been identified for oral candidiasis in SLE patients. A study recommends examining for oral candidiasis in those with active disease, proteinuria, high white blood cell count, and those taking prednisone, immunosuppressive agents or antibiotics.23 In our study, the third patient had proteinuria, was taking prolonged methylprednisolone and antibiotics for SLE, and had pulmonary tuberculosis. Therefore, he was at increased risk of opportunistic infection, such as oral or oropharyngeal candidiasis.
The fourth case demonstrated LE-non-specific aphthous oral ulcers. The white keratotic plaque observed at the lateral border of the tongue may indicate initial verrucous LE. This white plaque-like lesion may resemble homogeneous leukoplakia or reticular OLP.24 In 2005, the World Health Organization (WHO) defined leukoplakia as “a white plaque of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer”.25 In contrast, there were no changes in the tissue’s physical consistency, which is typically identified in leukoplakia.
In the present report, the diagnosis of SLE adhered to diagnostic criteria established by the SLICC. The disease activity was measured using the MEX-SLEDAI (Mexican Version of Systemic Lupus Erythematosus Disease Activity Index). The third and fourth cases had index scores of 17 and 2, respectively. A score greater than 5 indicates an active or flare condition, a score of 2–5 suggests the possibility of a flare, and a score of less than 2 represents an inactive condition or remission.26 Regrettably, the disease activity was not evaluated in the first and second cases.
The management of SLE involves the use of corticosteroids and an antimicrobial mouthwash to suppress the autoantibody response. Chlorhexidine digluconate (0.12%) was used as an antimicrobial in this case series due to its antiplaque properties, bactericidal action to prevent secondary infection of oral ulcers and promising antifungal benefits.27 The importance of a multidisciplinary approach and referral should be emphasized in the management of multi-organ SLE as it results in a shorter recovery time and improved patient quality of life.28, 29
The article described various oral lesions in adults and juveniles with SLE and their management. However, only 4 SLE cases were reported with limited follow-up on the patients’ systemic conditions. Additional research should be conducted with a larger sample size and a longer follow-up period.
Conclusions
In conclusion, oral manifestations in adult- and juvenile-onset SLE have a diverse presentation and may resemble lesions in other diseases. Additionally, they may occur as a manifestation of treatment received for the underlying systemic disease. The lesions presented in this report were mostly ulcerations, including aphthous-like ulcers, OLP-like lesions, palatal ulcers, erythema, and white keratotic honeycomb plaques, which may also indicate the underlying disease. Oral candidiasis may appear as a side effect of systemic condition treatment. A thorough evaluation of oral and systemic conditions is necessary for the accurate diagnosis and comprehensive management of patients with adult- and juvenile-onset SLE.
Ethics approval and consent to participate
The authors confirm that they have obtained all necessary patient consent forms. The patients were informed that their names and initials would not be published, and efforts would be made to conceal their identity.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
The patients have given their consent for the publication of medical images presented in the article.