Abstract
The available literature indicates that smoking causes quantitative and qualitative changes in saliva. However, there is a lack of studies summarizing the knowledge in this area, and there are no clear guidelines on the use of salivary biomarkers for assessing exposure to cigarette smoke (CS). The present work aimed to provide a systematic review of the literature regarding the influence of smoking traditional and electronic cigarettes, as well as heat-not-burn products, on salivary homeostasis. An electronic search of the literature from 1982 to 2023 was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Based on the inclusion criteria, 65 studies were used for the final review. Smoking traditional as well as electronic cigarettes negatively affects salivary biomarkers, including the salivary flow rate, pH, antibody titer, electrolyte concentration, microflora composition, redox balance, and inflammation, in terms of both quantity and quality. However, to date, only single salivary biomarkers have been compared in traditional and electronic cigarette smokers. It can be concluded that the salivary production rate, pH, microbiome, and cytokines can be used to assess exposure to CS smoke. There is a lack of convincing evidence to compare the toxic influence of traditional and electronic cigarettes on salivary homeostasis. Future experiments should include long-term randomized clinical trials on larger populations of smokers.
Keywords: smoking, saliva, biomarker
Introduction
Despite the implementation and popularization of anti-tobacco programs, addiction to smoking continues to be a global public health problem1 and is one of the primary causes of premature deaths, decreasing life expectancy by up to 8 years.2 Cigarette smoke (CS) contains over 5,000 harmful substances, 400 of which are scientifically proven carcinogens, such as formaldehyde, benzene and vinyl chloride,3 with the latest research demonstrating that the poisonous chemicals contained in cigarettes reach every organ in the human body.4 In addition to an increased risk of cancer, smoking raises the probability of stroke, respiratory diseases and inflammation, and weakens immune functions.5, 6 Smoking also adversely affects the oral ecosystem, the consequences of which are well-documented; they comprise relatively mild complaints (e.g., bad breath, tooth discoloration, and an increased accumulation of plaque and tartar), but also life-threatening diseases (including oral cancer).7, 8
The first protective barrier against the chemicals contained in cigarettes is saliva, which, under normal conditions, constantly moistens the mucous membrane and the teeth.9 Saliva is secreted from large and small salivary glands, and serves numerous functions in the body, including inhibiting the development of bacteria, and preventing the demineralization of hard tissues in the oral cavity and the formation of carious and non-carious cavities.10, 11 Furthermore, saliva is a source of enzymatic and non-enzymatic antioxidants, preventing redox homeostasis disorders in the oral cavity. It also enables the formation of a bolus, and initiates sugar and fat digestion. Reduced saliva secretion is a serious health problem leading to diseases within the oral cavity. It has been demonstrated that salivary homeostasis is influenced by various factors, including chronic diseases, such as obesity, insulin resistance, chronic kidney disease, congestive heart failure, psoriasis, stroke, and neurodegenerative diseases (Alzheimer’s disease and dementia).12, 13
Numerous studies have reported the negative impact of smoking on the salivary glands.14, 15, 16 Therefore, the assessment of salivary biomarkers may have a diagnostic value in monitoring the oral health consequences of smoking. Indeed, changes in the quantitative and qualitative composition of smokers’ saliva have been reported. However, no papers have summarized the knowledge in this area, and there are no clear guidelines on the use of salivary biomarkers for assessing exposure to CS smoke.
A “healthier” alternative to traditional cigarettes are electronic cigarettes (e-cigarettes; ECs),17 mechanical devices that heat special inhalation solutions and provide the user with an experience similar to traditional smoking.18, 19 E-cigarette liquid mainly consists of propylene glycol, glycerol, aromas, and nicotine,20, 21 although more detailed tests have confirmed the presence of formaldehyde, acrolein and heavy metals.22, 23 Although the common belief in a reduced harmfulness of ECs is now becoming increasingly controversial, the use of ECs, or so-called “vaping”, has become extremely popular among young people.24, 25 Aggressive marketing campaigns have also led to the “renormalization” of smoking,25 with the U.S. Food and Drug Administration (FDA) reporting a 900% increase in the use of ECs among high school students.26
E-cigarettes were introduced onto the market and patented in China in 2006.27 Due to their relatively short presence on the consumer market as compared to traditional cigarettes, the long-term effects of ECs are unknown and the knowledge about their short-term effects is still very limited. It is acknowledged that direct exposure to the EC aerosol may impair blood vessel and respiratory functions, but there is still a substantial gap in the knowledge on the effects of vaping on salivary homeostasis.28, 29 Also, there have been no reviews evaluating the influence of traditional cigarettes and the abovementioned new-era devices on various salivary parameters. Taking into consideration the harmful effects of smoking on the oral ecosystem, as well as the lack of non-invasive biomarkers to assess exposure to smoke, we conducted a systematic review of the literature on the salivary biomarkers in the smokers of traditional cigarettes, ECs and heat-not-burn products. We are the first to compare the available literature regarding the effects of smoking traditional and electronic cigarettes on the salivary secretion and composition.
Material and methods
Search strategy
The literature search was conducted up to September 3, 2023, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines,30 using the PubMed/MEDLINE, Scopus and Web of Science databases. We only evaluated international publications written in English. The available literature was browsed based on the following keywords: ‘cigarette and oral health’; ‘cigarette and saliva’; ‘smoking and saliva’; ‘smoking and salivary flow rate’; ‘smoking and salivary pH’; ‘smoking and salivary oxidative stress’; ‘smoking and oral microbiome’; ‘smoking and salivary immunoglobulins’; ‘smoking and oral inflammation’; ‘smoking and salivary minerals’; ‘e-cigarette and saliva’; ‘electronic cigarette and saliva’; ‘vaping and saliva’; ‘heated tobacco and oral health’; and ‘heated tobacco and saliva’. The inclusion and exclusion criteria are presented in Table 1.
Data extraction
Data pre-selection was carried out independently by 2 authors, based on the assessment of the titles and abstracts of the manuscripts, followed by a thorough review of the texts of the selected articles. Publications that met the inclusion criteria were considered for the review. In cases of doubt over the content of an article, the other authors were consulted. The reliability level of the researchers was determined using Cohen’s kappa coefficient (κ), which amounted to 0.92. All publications were evaluated in terms of methodology to ensure data quality, and the following variables were recorded: author(s); year of publication; study design; size of the study population; inclusion and exclusion criteria; duration of the study; and research results.
Results and discussion
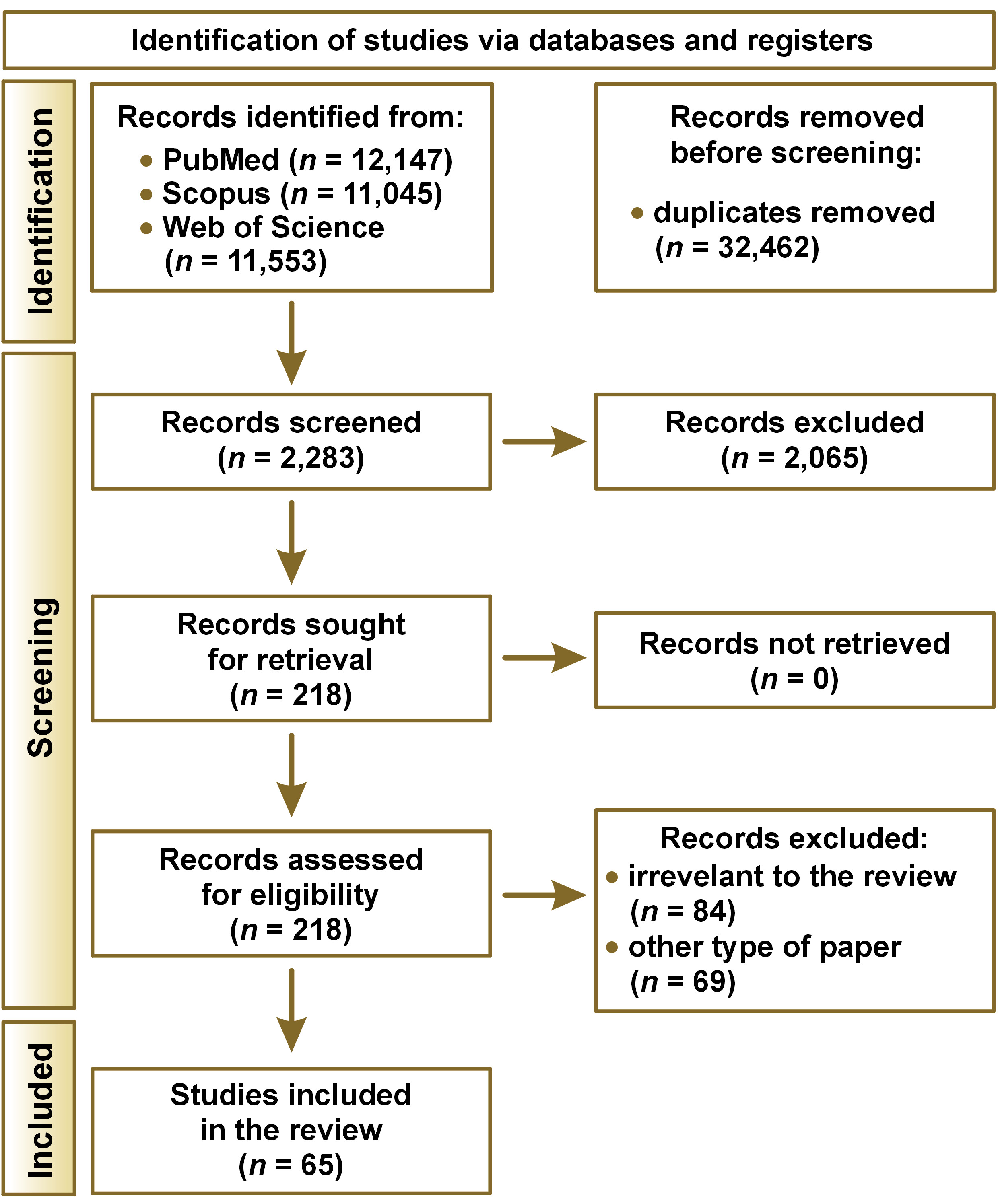
The literature search revealed 34,745 works from the PubMed/MEDLINE, Scopus and Web of Science databases, of which 32,462 were removed due to duplication. A total of 2,283 abstracts were read, with 218 meeting the inclusion criteria. Among the qualified articles, 153 either were irrelevant to the subject of our review or presented other type of paper than required, leaving 65 papers included. A PRISMA flow diagram presenting the search strategy is shown in Figure 1.
According to the Oxford Center for Evidence-Based Medicine (CEBM) 5-level classification scale of diagnosis, most of the studies presented the 3rd or 4th level of evidence (clinical control studies).31 Only 3 studies used a prospective cohort design.
Table S1 (the supplementary material is available on request from the corresponding author) shows the summarized quality assessment according to the Study Quality Assessment Tool guidelines issued by the National Heart, Lung and Blood Institute (NHLBI) within the U.S. National Institutes of Health (NIH).32 The critical assessment was performed by summing the points for each criterion with regard to the potential risk of bias (1 – low; 0.5 – indeterminate; and 0 – high). The most commonly encountered risks of bias were sample size justification, randomization and the blinding status of participants. Forty-seven studies (72.3%) were classified as having “good” quality (≥80% of total points), and 18 (27.7%) were classified as having “intermediate” quality (≥60% and <80% of total points).
The vast majority of studies included in the review focused on traditional cigarettes. Only 10 compared the toxicity of traditional vs. electronic cigarettes, using salivary biomarkers. The methodology and endpoints of all studies reviewed are presented in Tables S2–S7 in the supplementary material (available on request from the corresponding author).
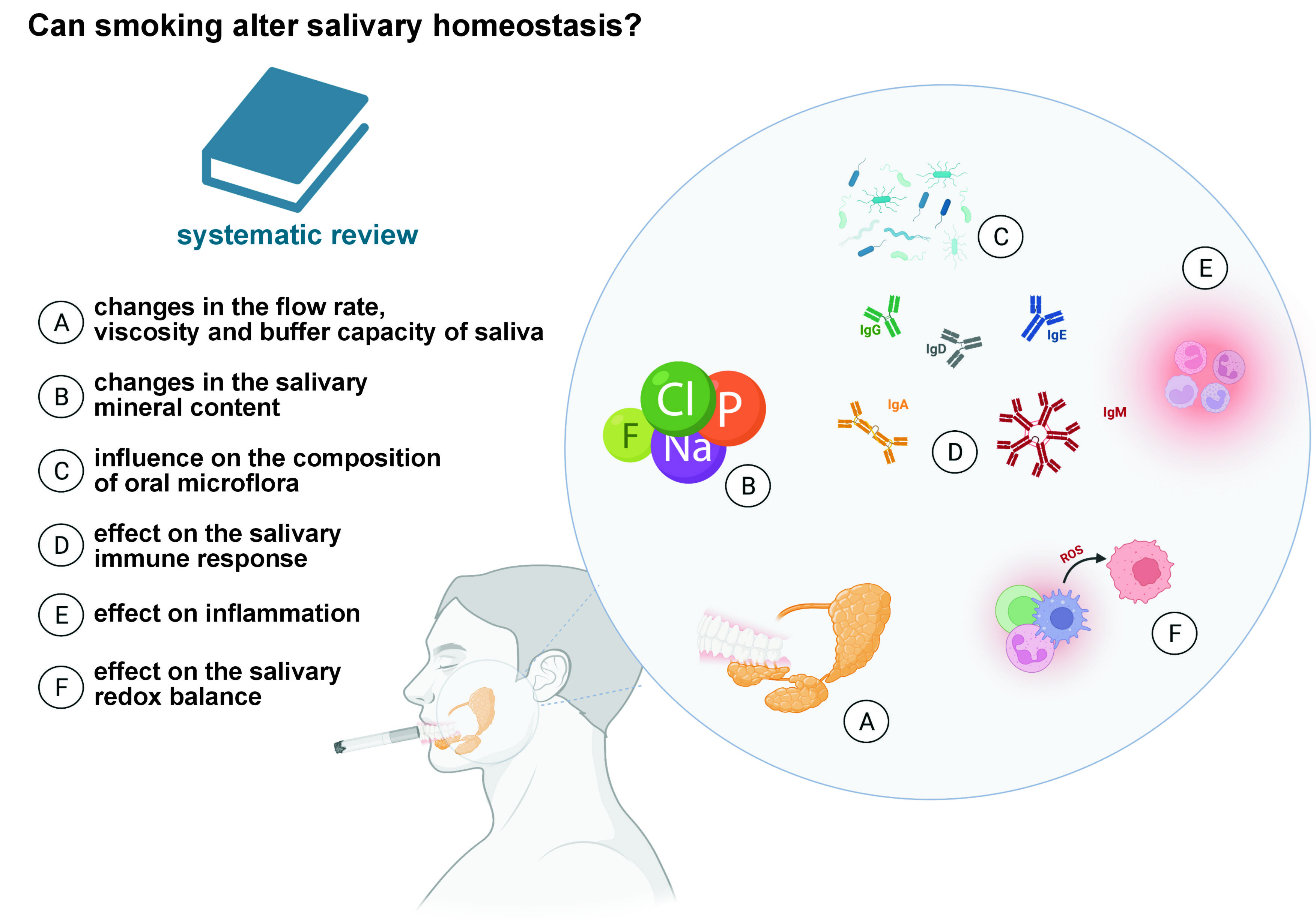
The multi-directional effect of smoking on various salivary parameters has been widely documented, and includes a decrease in the salivary flow rate (SFR) and pH, a reduction in the mineral content, and changes in microflora composition, and concentrations of antibodies, antioxidants and inflammatory mediators (Figure 2). A summary of the results of the systematic review is presented in Table 2.
Effect of cigarette smoking on the flow rate, viscosity and buffer capacity of saliva
The constant moistening of the oral cavity by saliva determines dental and periodontal health, with a proper SFR being a prerequisite for the continued remineralization of teeth, soft tissue protection and regeneration, antimicrobial effect of saliva, and the initial digestion of sugars contained in food.33, 34 Interestingly, smoking has been proven to reduce SFR. Confirming the previous observations,35, 36, 37, 38, 39, 40, 41, 42, 43 Singh et al. demonstrated a significant decrease in SFR in cigarette smokers.38 One of the mechanisms leading to a reduced SFR may be alterations in the tissue architecture of the salivary glands.44 In rats exposed to tobacco smoke, atrophic changes and inflammatory infiltration within acinar cells were observed in the parotid and submandibular glands.44 In secretory cells, the extracellular matrix is enlarged and fibrotic due to an increase in type III and type I collagen fibers. Meanwhile, structural alterations in glandular cells disturb the microenvironment of the salivary gland stroma and may be responsible for reduced salivary secretion. Increased type I collagen density can also be observed in the stroma of malignant neoplasms.45 A reduced SFR induces symptoms such as dry mouth (including difficulty in swallowing and speaking), impaired taste perception, and sore tongue and lips (which become dry and cracked), contributing to increased thirst and discomfort, and may also explain an increased incidence of dental caries, periodontal disease and fungal infections in smokers.46, 47
Several authors also reported increased saliva secretion after the exposure of the oral mucosa to CS.48, 49 An increased SFR in smokers may be caused by the presence of nicotinic receptor agonists (nicotine and cytosine) in cigarettes, triggering the chemical stimulation of taste receptors and stimulating the salivary glands.50, 51 In addition, nicotinic agonists lead to an increased release of norepinephrine and acetylcholine from autonomic nerve endings via the activation of the nicotinic receptor subtype α3β4.51 Those who have started smoking recently may experience a temporary increase in salivary gland activity in response to the stimulation of the nerve endings of taste receptors by alkaloids contained in tobacco smoke, followed by a gradual quieting of the receptors.52 Another explanation for increased salivation in smokers could be found in the “irritation” theory.53 Smoking can significantly increase salivation by the parotid glands as compared to the state before smoking due to the exposure of the oral mucosa to the toxic components of CS (irrespective of the smell), inducing the secretion of stimulated saliva.54 Responding to irritation by increasing saliva production is consistent with its protective function. To protect the oral mucosa, toxic and damaging factors are diluted with an increased amount of saliva, and rinsed. Although smoking is considered pleasant for smokers and unpleasant for non-smokers, the response to irritant stimuli should be greater in non-smokers.54, 55
In contrast, Petrušić et al. found no significant difference in the amount of unstimulated and stimulated saliva between smokers and non-smokers (though SFR was lower in smokers).56 The authors concluded that SFR in smokers decreased with smoking duration, and the amount of the secreted saliva did not depend on the number of cigarettes smoked per day. They also observed a change in the quality of salivary gland secretion.56
In the majority of nicotine addicts, saliva is reported to be thick and viscous, while in non-smokers, it is thinner.36, 41 In a study by Kusumaningrum et al., 57% of smokers demonstrated moderate saliva viscosity, 43% were included in the poor saliva viscosity category, and no participants demonstrated normal saliva viscosity.36 In the group of non-smokers, 75% of participants had normal saliva viscosity, and 25% had moderate saliva viscosity, indicating that no subjects had poor saliva viscosity.36 An increase in saliva viscosity due to smoking may be connected with a high sensitivity of the parotid glands to tobacco smoke toxins.57, 58 The parotid glands are responsible for producing watery, serous saliva, and its absence is compensated by the submandibular and sublingual salivary glands, which secrete thicker saliva with a high mucin content.59, 60 Thick saliva does not effectively moisten the oral cavity, which can cause symptoms similar to those of decreased saliva secretion, and lead to a subjective feeling of dryness in the mouth and intensified symptoms of dental caries.61 Furthermore, people with abnormal saliva density often complain about impaired swallowing and difficulty in forming a food bolus.
In addition to the proper flow rate and viscosity of saliva, an important factor in maintaining healthy teeth and periodontium is its buffer capacity.62 The stimulated saliva secreted by the parotid glands contains significant amounts of bicarbonate buffers that maintain a normal pH, and neutralize acids from food and those produced by cariogenic bacteria.63, 64 Factors that reduce SFR also decrease the buffer capacity of saliva and increase the risk of caries. Indeed, patients with a low SFR had lower bicarbonate concentrations, and this relationship correlated with an increased prevalence of caries and cavities of non-carious origin.36, 41 In a study by Singh et al., the mean salivary pH reached 6.30 ±0.36 in smokers and 7.10 ±0.24 in non-smokers.38 After measuring the salivary pH of smokers, Saputri et al. found that 67.5% of them had pH < 6.7, for 32.5%, it ranged from 6.7 to 7.4, while pH > 7.4 was not recorded in any participant.37 Voelker et al. showed no association between the nicotine levels in cigarettes and the pH of saliva.41 In both studies, a decrease was observed in the pH values along with lower values of SFR.37, 41 Furthermore, it was proven that the saliva buffering response as a consequence of drinking acidic carbonated beverages was 20% lower in smokers than in non-smokers42 (Tables S2–S4, available on request from the corresponding author).
Effect of smoking on the salivary mineral content
Saliva contains a number of electrolytes, the final content of which is determined through a process that takes places at the level of the striated ducts of the salivary glands.65 Although there is little data on the relationship between oral health and ions present in saliva, the salivary electrolyte levels are determined by general health.66, 67, 68, 69, 70, 71, 72 Calcium (Ca) and phosphorus (P) ions, in addition to participating in the remineralization of dental hard tissues (along with sodium (Na) and magnesium (Mg) ions), contribute to plaque mineralization and tartar formation.73, 74, 75, 76 A similar function is performed by fluorine (F), which binds to the amino groups in hydroxyapatite and replaces hydroxide ions to form fluorapatite, which is more resistant to acids produced by bacteria and contained in food.77 A few authors attempted to determine the salivary electrolyte profile of smokers, with most studies indicating no effect or an insignificant decrease in the electrolyte content. Kolte et al. investigated the relationship between smoking and the salivary levels of Ca, Mg and P, and found decreased concentrations in the smoking group as compared to non-smoking controls.66 Similarly, Sewón et al. revealed a significantly decreased Ca content in the saliva collected from 90 smokers.69 Changes in the salivary concentrations of Ca, Mg and phosphate ions, along with decreased plaque pH, may be responsible for impaired enamel mineralization, which facilitates tartar accumulation in smokers. Other studies reported a decreased salivary concentration of zinc (Zn), serving as a cofactor for superoxide dismutase (SOD).67, 78 Superoxide dismutase is a vital component of the body’s antioxidant mechanisms responsible for catalyzing the dismutation reaction of superoxide radicals to hydrogen peroxide and oxygen. Thus, a decrease in the salivary Zn concentration may partially explain reduced SOD activity and increased oxidative stress in smokers (Table S5, available on request from the corresponding author).
Effect of smoking on oral microflora composition
Several studies demonstrated that smokers were less diligent in their oral hygiene regime than non-smokers.79, 80, 81 The level of oral hygiene is determined by the number of caries-related bacteria, including variable streptococci and lactic acid bacteria, the levels of which can be measured in saliva.82, 83, 84, 85
In a study by Heintze, smokers had a significantly higher number of lactobacilli and Streptococcus mutans, considered major cariogenic bacteria.86 The count of lactobacilli in saliva correlated positively with the number of cigarettes smoked per day, and about 40% of smokers had the count of S. mutans more than doubled as compared to non-smokers.86 Saliva plays a critical role in preventing bacteria from adhering to tissues. Low pH and flow rate of saliva, as well as its low buffer capacity, may additionally favor the development of lactic acid bacilli. In addition, it was observed that smoking correlated with the number of commensal Candida albicans.87 The study indicated that smoking traditional cigarettes inhibited the growth of Gram-positive cocci, which delay the colonization of carious bacteria, including the pioneer species of Neisseria, and CS promoted the growth of Gram-negative microorganisms.88, 89, 90 On the other hand, Nakonieczna-Rudnicka and Bachanek reported no significant relationship between the number of S. mutans and lactobacilli in saliva and smoking duration or the number of cigarettes smoked per day.91
In the past, it was believed that the sugar content in cigarettes was the main contributor to the growth of caries-related bacteria, and that the chemical compounds contained in cigarettes did not play a substantial role. However, the current prevailing assumption is that nicotine stimulates the development of cariogenic microflora.82 Nicotine is responsible for an increased adhesion of planktonic bacteria to the tooth biofilm and its thickening due to an increase in the synthesis of extracellular polysaccharides (EPS), glucosyltransferase (Gtf) and glucan-binding protein (Gbp) at the mRNA and protein levels.92, 93 The influence of nicotine on the activity of lactate dehydrogenase (LDH) was also assessed.94 Lactate dehydrogenase is an enzyme that catalyzes the last step in the bacterial glycolytic pathway, converting pyruvate to lactate.95 Although nicotine does not directly affect LDH, it indirectly enhances its activity by increasing the total amount of bacteria.93 As such, more lactic acid may contribute to the risk of caries (Table S6, available on request from the corresponding author).
Effect of smoking on the salivary immune response
Saliva has a defensive function, since it contains several immunoglobulins (Ig) – IgA, IgG, IgM – and antioxidant factors (lysozymes).96, 97 Secretory IgA (SIgA) is the predominant immunoglobulin in salivary gland secretions,98 and is the first line of the host defense against pathogens that colonize or attack the surfaces covered by external secretions.97, 99, 100 The primary function of SIgA is to limit microbial adherence and the penetration of foreign antigens into the mucosa. Naturally occurring SIgA antibodies have been detected in saliva. Although the role of SIgA in the colonization and regulation of the native bacterial flora is still questionable, less abundant salivary IgG and IgM limit bacterial adherence to enamel and cheeks.97 The effect of smoking on the levels of salivary immunoglobulins has been widely documented,101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112 with most studies reporting a significant reduction in the SIgA content under the influence of smoking. In the work of Giuca et al., the concentrations of IgA, IgG and IgM antibodies decreased in smokers by 91%, 99% and 83%, respectively, as compared to the non-smoking control group.104 On the other hand, Tarbiah et al.101 and Engström and Engström110 observed an increased IgA antibody concentration in the saliva of smokers.101, 110 In contrast, Olayanju et al. revealed a decreased IgM content, with no effect on the amount of IgA and IgG.108 Abnormal salivary immunoglobulin levels may partially explain an increased incidence of periodontal disease in smokers. Both the local irritant effects of tobacco smoke and exposure to nicotine and its metabolites can disrupt humoral immune mechanisms in the oral cavity. In smokers, there are abnormalities in the response to the antigens of plaque bacteria, involving impaired chemotaxis, phagocytosis and neutralization of bacterial enzymes and toxins.100, 113 Of particular importance is a decrease in salivary IgG, involved in the activation of the complement system and the removal of Aggregatibacter actinomycetemcomitans, responsible for periodontitis progression.110
Nagler determined the salivary immunological profile in compulsive smokers and its potential correlation with the development of oral cancer.3 Biochemical and immunological analyses showed a significant reduction in the IgG antibody level, as well as increased albumin content and activity of amylase, LDH, matrix metalloproteinase 2 (MMP-2), and MMP-9 by 61%, 86%, 65%, 35%, and 55%, respectively. Matrix metalloproteinases are matrix-degrading enzymes secreted during the migration of epithelial cells to the underlying connective tissue, with MMP-2 and MMP-3 shown to play a central role in oral cancer invasion and metastasis.3 Therefore, there is an imbalance between oral protective and procarcinogenic factors in smokers. Studies suggest an increased rate of epithelial cell exfoliation into the oral cavity, causing many compounds to pass from serum into saliva through the compromised oral and gingival mucosa.111
Although the exact mechanisms of the influence of CS on the body are still unknown, adverse reactions to the cellular immune response are associated with exposure to aromatic compounds, heavy metals, nicotine, and other toxins.53, 114 Indeed, long-term exposure to nicotine has been shown to suppress the cellular response through reduced antibody production, disrupted antigenic signaling in T lymphocytes and T cell anergy115 (Table S6, available on request from the corresponding author).
Effect of smoking on the salivary redox balance
Cigarette smoke is a mixture of thousands of substances responsible for generating reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as benzene, 2-napthylamine, cadmium (Cd), and benzopyrene. Free radicals are highly reactive species that readily oxidize cellular biomolecules (i.e., lipids, proteins and nucleic acids).116, 117 Situations in which antioxidant mechanisms are unable to cope with the neutralization of ROS/RNS lead to oxidative stress,118 which disrupts cell metabolism, and ultimately leads to cell death by apoptosis or necrosis.119 Saliva is the first biological fluid whose antioxidant systems oppose the free radicals produced while smoking. Several studies have indicated that smoking affects the antioxidant barrier of saliva.44 Fujinami et al. showed that passive exposure to tobacco smoke resulted in a significant reduction in the activity of salivary peroxidase (Px), catalase (CAT) and α-amylase.44 Interestingly, changes in salivary antioxidant enzymes were accompanied by histological changes within the salivary glands. After 30 days of the experiment, the researchers observed an increase in the interlobular duct area, part of the striated duct, and vacuolar degeneration in the entire CS-exposed parotid gland, as well as in the central part of the submandibular gland. The salivary glands are highly sensitive to oxidative cellular damage, and since oxidative stress is a key factor responsible for impaired secretory function of the salivary glands, hyposalivation in smokers may be caused by disturbances in redox homeostasis.44 Nevertheless, this hypothesis requires further investigation.
Depletion of antioxidant enzymes has also been observed in clinical trials, with many studies clearly showing that cigarette smoking is accompanied by a decrease in the activity of SOD, CAT and Px (the latter by up to 76%).44, 120, 121, 122, 123, 124, 125, 126 Enzymatic antioxidants are the first line of defense, preventing the reaction of free radicals and their derivatives with salivary biomolecules. When the activity of Px and CAT is reduced, intoxication with hydrogen peroxide in the oral cavity is significantly reduced.123, 124, 125, 126, 127, 128 In the presence of transition metal ions, hydrogen peroxide initiates the Fenton and Haber–Weiss reactions, leading to the formation of a highly reactive hydroxyl radical (•OH).119 The reactive oxygen species generated during smoking are also responsible for a decrease in the concentrations of reduced glutathione (GSH) (probably as a result of combining cysteine contained in GSH with aldehydes, which are components of CS), as well as uric acid (UA) and LDH.129, 130 Uric acid is the major antioxidant of saliva, determining up to 70–80% of its antioxidant capacity. Some other low-molecular-weight hydrophilic antioxidants, such as vitamin C, also play a key role in counteracting salivary oxidative stress. Smokers have significantly lower levels of salivary vitamin C.131 The reason for the abovementioned phenomenon is multifactorial. Firstly, smokers consume less dietary antioxidants and absorb them less efficiently than non-smokers. Secondly, the protective effect of antioxidants is more rapidly worn down in smokers by continuous exposure to ROS.131 Therefore, it is not surprising that in people exposed to CS, a significant decrease in salivary total antioxidant capacity (TAC) is observed.129, 131, 132, 133, 134
Salivary TAC characterizes the resultant ability of saliva to scavenge oxygen free radicals. It is well known that the evaluation of salivary TAC provides much more information than the assessment of individual antioxidants separately, as the synergistic effects of ROS scavengers are often observed.62 Bakhtiari et al. demonstrated a 29% drop in TAC in smokers as compared to the control group.131 In another study, Nagler et al. showed that salivary TAC (the ImAnOx® assay) and SOD activity were decreased in smokers by 32% and 12%, respectively.3 A consequence of a diminished antioxidant barrier is enhanced oxidation of salivary lipids, proteins and nucleic acids. In a study by Demirtaş et al., the concentration of malondialdehyde (MDA) in the saliva of smokers was significantly higher as compared to the control group and the group of passive smokers,135 which partially agreed with the results of other research groups. Evidence showed that salivary MDA has a pathological role in multi-step oral carcinogenesis and cancer progression. Metgud and Bajaj observed a significant elevation in the salivary MDA levels, which progressed from a healthy control group, through a precancerous state, to individuals with squamous cell carcinoma (SCC),136 while Nagler et al. showed higher salivary total protein carbonyls (by 126%) in the smoking group as compared to non-smokers.3 Reznick et al. also noted significantly increased carbonylation of salivary proteins in heavy smokers and non-smokers exposed to cigarette smoking one time.126
On the other hand, Kanehira et al.137 and Baharvarnd et al.122 reported increased SOD activity in the saliva of cigarette smokers. It can be speculated that the enhancement of the salivary antioxidant barrier is an initial adaptive response to CS-induced ROS overproduction in novice smokers. With an increasing duration of smoking, the antioxidant reserves of saliva may become depleted and the oxidative injury to saliva biomolecules increases. Further research is needed on the relationship between smoking duration, the number and type of cigarettes smoked and salivary redox homeostasis.
Interestingly, CS-induced oxidative stress does not decrease after the oral administration of antioxidant compounds, including a strong ROS-scavenger vitamin C.138 Even after 4 weeks of vitamin C supplementation, smokers did not achieve an increase in salivary TAC to the levels observed in the control group.138 Azimi et al. investigated the effect of a 3-week consumption of green tea on the salivary antioxidant barrier in heavy smokers, occasional smokers and non-smokers.139 At the beginning of the experiment, the antioxidant capacity of saliva differed between the 3 groups, reaching the highest values in the control group and the lowest in the heavy smokers group. After 7 days of green tea consumption, there was no significant difference between occasional smokers and non-smokers. Heavy smokers were characterized by a significantly higher TAC as compared to the other 2 groups, with the same relationship occurring after 14 and 21 days of the study. The TAC level showed an upward trend during the experiment, and the antioxidant capacity on the 21st day of green tea drinking was significantly higher as compared to the measurement made at baseline. Nevertheless, the TAC values in the smoking group did not come close to those in the control group. The beneficial effect of green tea may be explained by a high content of catechins, monomeric polyphenols that have a strong antioxidant effect, supporting ROS sweeping. Interestingly, early studies showed that green tea catechins could be detected in saliva even after a vigorous rinsing of the mouth139 (Table S7, available on request from the corresponding author).
Effect of smoking on the salivary inflammation
Salivary redox homeostasis is inextricably linked to oral inflammation, with smoking shown to interfere with the synthesis and secretion of inflammatory mediators in saliva. Cigarette smoke increases the release of tumor necrosis factor alpha (TNF-α), interleukins (IL) IL-1, IL-6 and IL-8, and granulocyte–macrophage colony-stimulating factor (GM-CSF).140, 141 The main source of inflammation in the mouth comes from activated macrophages, which contribute to the elimination of bacteria and viruses through phagocytic and cytotoxic activity. Their activation results in increased production of proteolytic enzymes, cytokines, chemokines, growth factors, and oxygen free radicals.140 However, macrophages also show high reactivity to CS. In smokers, macrophages produce more TNF-α, IL-1 and IL-6 than in non-smokers.142 In addition to changes in macrophage morphology (e.g., cytoplasm thickening and its higher density), these cells also show higher CD14 expression, stronger inhibition of lymphocyte and NK (natural killer) proliferation, and less ability to phagocytose microorganisms. Cigarette smoke also changes the T helper (Th)1/Th2 cell ratio.143
Exposure to CS generally reduces the ability of the circulating dendritic cells to present antigens to lymphocytes. Rahimi et al. found a significant increase in the levels of interferon gamma (IFN-γ) and IL-2 in the saliva of smokers as compared to non-smokers.144 Thus, CS mainly affects the balance of cytokines produced by Th cells. Importantly, the concentrations of these cytokines increased with smoking duration.144 Mokeem et al. demonstrated significantly higher levels of pro-inflammatory IL-1β and IL-6 in the saliva of cigarette smokers.145 It is worth mentioning that IL-1β in saliva is associated with active smoking, regardless of the number of cigarettes smoked, in contrast to the level of TNF-α, the concentration of which positively correlates with the number of cigarettes smoked daily. In a study by Nishida et al., the analysis of salivary cytokines in workers aged 18–62 years showed that the mean level of IL-1β in saliva was significantly higher in passive smokers (190 pg/mL) than in non-smokers (164 pg/mL); however, there was no difference between passive and active smokers (167 pg/mL).146 Moreover, the levels of other biomarkers, such as prostaglandin E2 (PGE2), MMP-9, lactoferrin, albumin, and aspartate aminotransferase (AST), were significantly lower in active smokers.146
Nicotine, hydroquinone and carbon monoxide are the main toxins responsible for the immunosuppressive and pro-inflammatory effects of CS. Nevertheless, nicotine can also reduce IL-6 production. Rodríguez-Rabassa et al. observed significant differences in the expression of salivary interleukins (↑ IL-2, ↑ IL-4, ↓ IL-5, ↓ IL-10), adrenocorticotropic hormone (ACTH) (↑), insulin (↓), and leptin (↓) in smokers as compared to non-smokers.89 The effects of smoking on salivary cytokines and chemokines are extensively studied in relation to periodontitis. There are a few reports on the concentrations of salivary cytokines in the context of smoking and the immune response of healthy participants. For example, in a study by Rathnayake et al., a group of healthy smokers showed a significantly reduced salivary IL-8 concentration as compared to non-smoking controls.147 Based on these data, it can be concluded that smoking has the potential to suppress the host defense system and promote the progression of periodontal disease. The effect of smoking on salivary cytokines is likely to vary with regard to age, the characteristics of the host defense system and periodontitis progression.147 Ageing is characterized by quantitative and qualitative changes in the immune system (i.e., increased levels of pro-inflammatory cytokines and decreased levels of anti-inflammatory cytokines). Unfortunately, the exact mechanisms through which tobacco smoke affects oral homeostasis have not yet been thoroughly investigated (Table S6, available on request from the corresponding author).
Effect of smoking electronic cigarettes on the salivary secretion and composition
The effect of EC smoking on saliva remains unclear.148 The first smokeless cigarette was patented by Herbert A. Gilbert in the state of Pennsylvania, USA, in 1967. However, the peak of EC popularity was in 2004, when Hon Lik, a Chinese pharmacist, modernized the original version of ECs and started distributing them in his local market.149 Undoubtedly, due to the short presence of ECs on the consumer market as compared to tobacco cigarettes, their long-term effects on human health cannot be determined. There are also scarce literature sources available on the effects of vaping on the secretion of saliva. Therefore, further research is necessary to assess the impact of EC use.
Several publications have evaluated the salivary pH and flow rate in EC smokers. Lestari et al. investigated the direct effect of smoking ECs on SFR and the salivary pH, and their results clearly indicated a significantly lower pH in EC smokers as compared to non-smokers, but no significant difference in the SFR levels between the 2 groups.150 However, the methodology of their experiment did not include the collection of saliva from traditional cigarette smokers and the comparison of the traditional smokers with the vaping group.150 This relationship was taken into account by Mokeem et al., whose results did not reveal any changes in the SFR values between the 3 study groups (EC smokers vs. traditional smokers vs. non-smokers).145
In research performed by Cichońska et al., pH was lower, and total protein, Ca and phosphate concentrations were higher in EC users in than non-smokers.151 The saliva of EC users showed changes in its physicochemical composition as compared to traditional smokers and non-smokers, but significant differences were only observed for the Ca concentration.151
An imbalance in oral microbiota was suggested in EC smokers.152, 153 To measure the effect of vaping on oral microbiota, a team of researchers assigned around 100 volunteers into one of the 3 groups – traditional smokers (an average of half a pack a day), EC smokers (half an EC a day) and non-smokers.153 The first finding was that the rates of periodontal disease severity (periodontal pocket depth (PPD) and bleeding on probing (BOP)) among EC smokers were significantly lower (42.5%) than in traditional smokers (72.5%), but were considerably higher as compared to non-smokers (28.2%). The second conclusion was that EC smokers showed a dysbiosis of oral microbiota comparable to that caused by traditional smoking. Their saliva was generally richer in bacteria as compared to non-smokers and showed the proliferation of various species harmful to oral health. Furthermore, human cells exposed to the EC aerosol showed increased susceptibility to bacterial infections as compared to cells exposed to clean air. The results of this study (conducted on humans (in vivo) and cells (in vitro)) confirm that vaporization disturbs the balance of oral microbiota and increases susceptibility to infections.153 Cichońska et al. investigated changes in the antibacterial properties of the saliva of EC smokers.154 A total of 120 subjects (40 EC smokers, 40 traditional smokers and 40 non-smokers) where recruited to the experiment which included the assessment of the lysozyme, lactoferrin and IgA content in saliva. The study revealed that EC users had lower salivary lysozyme and lactoferrin levels as compared to non-smokers, although no difference in the IgA antibody concentration was observed between the 2 groups. The results clearly demonstrated that ECs decrease the antimicrobial capacity of saliva. Nevertheless, in the group of tobacco cigarette smokers, all the studied parameters had lower values as compared to the EC group.154
The latest study by Cichońska et al. indicates that EC smokers exhibit a salivary redox imbalance to the same extent as traditional cigarette smokers, expressed in a lower TAC value in the saliva of EC smokers as compared to non-smokers.155 Although the results indicated only a slight effect on the salivary UA concentration in traditional cigarette smokers as compared to non-smokers, the UA concentration in EC users was higher than in traditional cigarette smokers and non-smokers. Disturbances in the salivary antioxidant potential may be closely related to an increased incidence of oral cancer in EC smokers, since oxygen free radicals can induce DNA damage, which can lead to neoplastic transformation.155
A study by Mokeem et al. suggested less harmful effects of smoking ECs, with the whole saliva concentrations of the pro-inflammatory cytokines IL-1β and IL-6 being significantly higher in traditional smokers than in EC users and non-smokers.145 Interestingly, the interleukin levels in EC smokers reached similar values to those of non-smokers.145 On the other hand, Ye et al. found elevated levels of the inflammatory marker PGE2 in traditional smokers as compared to EC users and the non-smoking group.156 In contrast, Singh et al.157 and Faridoun et al.158 showed a significant increase in IL-1β in EC users. In addition, a higher concentration of transforming growth factor beta (TGF-β) was found in EC smokers.158 In cancers, TGF-β expression significantly increases with the increasing tumor grade, suggesting a close relationship between this cytokine and malignant tumor changes. On the other hand, TGF-β is a cytokine released in inflammation and its level is increased in vitro by exposure to tobacco smoke. Notably, tumor development may be promoted by the altered TGF-β signaling pathway. Some previous studies reported increased levels of TGF-β in growing tumors and highlighted the prognostic properties of this cytokine.159
In addition to ECs, there are also heat-not-burn products on the market, which are attracting interest, especially from young smokers. According to the manufacturers, heating rather than burning tobacco is supposed to ensure less bodily exposure (including the mouth) to the harmful components of CS. Unfortunately, only one study evaluated salivary biomarkers in the smokers of heat-not-burn products. Mori et al. showed that using heat-not-burn products leads to a reduction in the secretion of unstimulated saliva.160 They also found reduced concentrations of anti-inflammatory lactoferrin and lysozymes in smokers’ saliva. These results suggest an adverse effect of heating tobacco on oral immune defense.160
Despite studies reporting decreased concentrations of serum antioxidants in EC users (↓ UA, ↓ carotenoids (including lutein), and ↓ α- and β-carotene) or increased levels of lipid peroxidation biomarkers in blood (↑ MDA), there is no similar data available in the context of saliva.161 Therefore, it is difficult to determine whether traditional smoking has different effects on the oral cavity than ECs or heat-not-burn products, making further research in this area necessary (Tables S2, S4–S7, available on request from the corresponding author).
Limitations and the next steps
To our knowledge, this study is the first systematic literature review comparing the effects of traditional cigarettes, ECs and heat-not-burn products on selected saliva biomarkers. Unfortunately, the study protocol was not registered, and due to the high heterogeneity of the studies, we could not use advanced statistical methods to analyze the data, which would have increased the quality of the conclusions drawn. The inclusion and exclusion criteria were not precisely specified in all studies, and the sample size was not set a priori. Also, standardized saliva sample collection and processing procedures were not used in the analyzed studies. In addition, a variety of biochemical methods were used to assess the salivary analyte concentrations, making an objective data comparison difficult. It should also be noted that individual quantitative/qualitative changes in the saliva of smokers, in addition to the effect of tobacco smoke, may be due to other factors, such as concurrent alcohol dependence, concurrent periodontal disease, the use of prosthetic restorations, and poor oral hygiene, which may contribute to the development of the pathologies discussed. Similarly, the innate/hereditary predispositions of smokers may influence the occurrence of pathologies in saliva in response to the aforementioned stimuli. Therefore, future experiments should include randomized clinical trials on larger populations of smokers. With regard to oral and general health, long-term observations are essential. To date, the diagnostic usefulness of saliva for assessing exposure to CS has only been assessed for selected biomarkers, which also indicates the need for further research.
Conclusions
Smoking has been a substantial public health problem for years, and numerous studies have demonstrated that smoking tobacco causes damage to almost every organ of the body, including significant impairment of the functions of the oral cavity. The exposure of the oral cavity to tobacco smoke may result in reduced saliva secretion and pH, as well as decreased immune and antioxidant salivary defense. Smoking impairs the antimicrobial properties of saliva, and increases susceptibility to caries and oxidative damage caused by the overproduction of oxygen free radicals, which is reflected in the quantitative and qualitative composition of saliva.
Some salivary biomarkers can be used to assess exposure to tobacco, including SFR, salivary pH, microbiome, and cytokines. In addition, salivary biomarkers can help assess the development of caries, the progression of periodontal disease, oral candidiasis, and oral cancer. However, the standardization of saliva sampling and processing procedures is required, as well as the development of the reference values for all salivary biomarkers.
E-cigarettes, supposedly less harmful than tobacco ones, were intended to replace the latter, and their popularity is still growing. Unfortunately, since they have only been available for a relatively short period, most of their short- and long-term effects are still unknown. Several publications report that, similar to traditional cigarettes, ECs may reduce the salivary pH or increase the levels of pro-inflammatory markers in saliva. This paper is the first systematic literature review comparing the effects of traditional cigarettes, ECs and heat-not-burn products on selected salivary biomarkers. However, there is a lack of convincing evidence to compare the toxic influence of traditional and electronic cigarettes on salivary homeostasis, with only individual salivary biomarkers in traditional and EC smokers assessed so far. Further research is necessary to fill the knowledge gap on the effects of ECs on the oral cavity. Future experiments should include randomized clinical trials on larger populations of smokers. With regard to oral health, long-term observations are essential. Furthermore, it is necessary to inform the public about the potential adverse effects of smoking in order to raise awareness of possible oral health consequences.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.