Abstract
Background. Single-nucleotide polymorphisms (SNPs) in the IKKβ gene have been associated with susceptibility to various inflammatory illnesses, including periodontitis.
Objectives. The aim of the present study was to investigate the association between IKKβ SNPs (at rs17875746 and rs12676482) and periodontitis in an Iraqi Arab population.
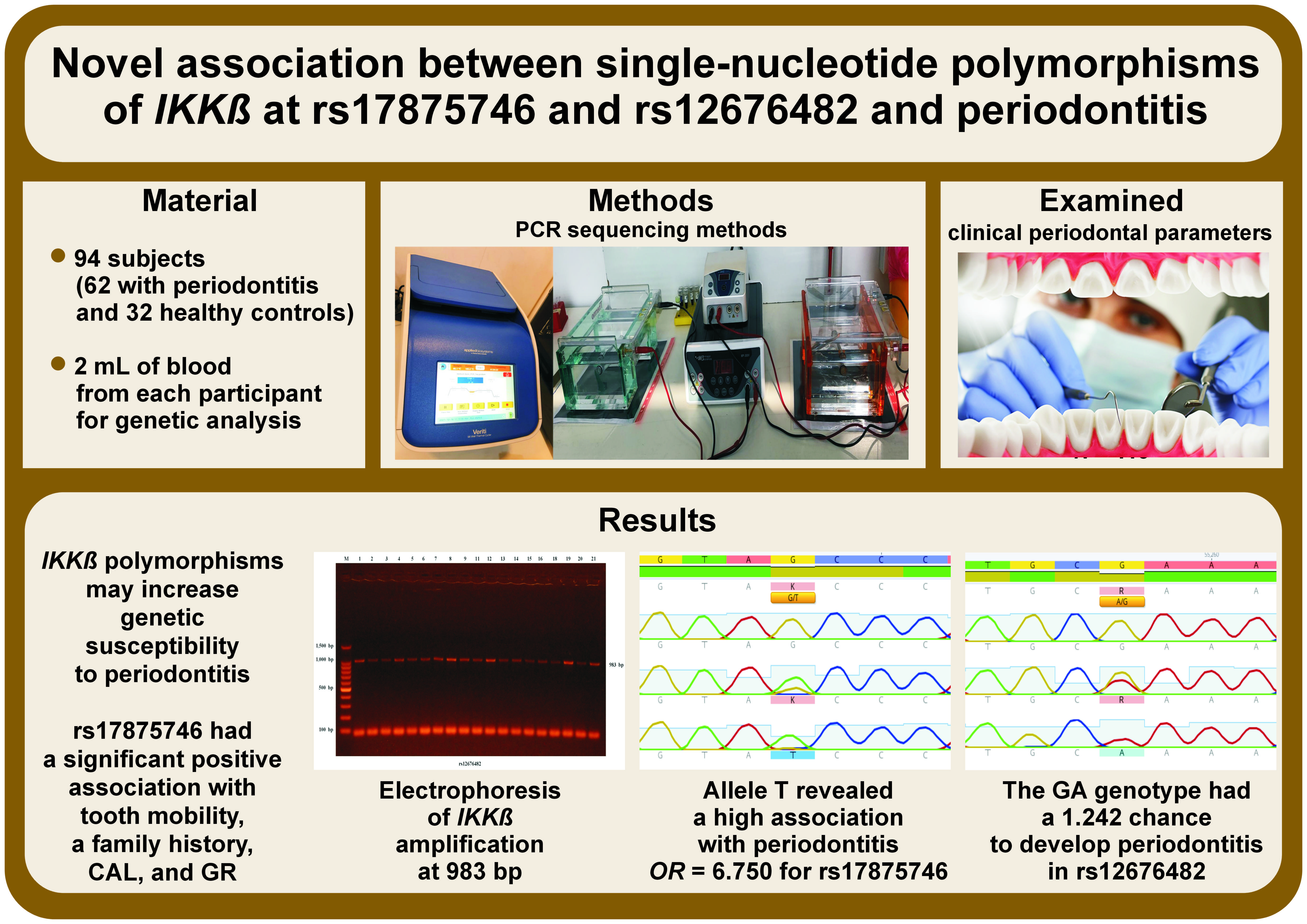
Material and methods. In this case–control study, 94 Iraqi volunteers were split into 2 groups, with the case group including 62 periodontitis patients (37 men and 25 women) and the control group including 32 racially matched healthy people (19 men and 13 women). Periodontal parameters were recorded for each individual. Then, 2 mL of venous blood was taken from each participant to isolate their genomic DNA. In particular, the genotyping of rs17875746 and rs12676482 in IKKβ was performed with the use of the polymerase chain reaction (PCR) sequencing methods.
Results. The effect of the distribution of IKKβ SNPs on periodontitis was assessed by counting the odds ratio (OR), which was 5.264 for rs17875746 and 0.900 for rs12676482. Surprisingly, allele T revealed a significantly higher association with periodontitis for rs17875746 (OR = 6.750) than allele G (p = 0.038). Overall, the GT genotype in rs17875746 had a higher chance of developing the disease (OR = 3.321) as compared to other genotypes. Meanwhile, the GA genotype in rs12676482 had a higher chance of developing the disease (OR = 1.242) as compared to other genotypes. In addition, rs17875746 showed a significant positive association with tooth mobility, a family history, clinical attachment loss (CAL), and gingival recession (GR) in the study groups.
Conclusions. The IKKβ polymorphisms may increase genetic susceptibility to periodontitis in Iraqi Arab patients.
Keywords: IKKβ, periodontitis, single-nucleotide polymorphisms
Introduction
The breakdown of periodontal attachment and alveolar bone resorption are characteristic of the chronic inflammatory disease known as periodontitis. The disease is initiated by microbial plaque and exacerbated by risk factors, such as systemic diseases, genetic factors and environmental factors.1 It has approx. 50% heritability, meaning that genetic variance influences people’s susceptibility to this disease.2 Specifically, genetic polymorphisms in the molecules involved in the pathogenesis of periodontitis have been linked to an increased risk of periodontitis at an individual level.3, 4
The transcription factor nuclear factor-kappa B (NF-κB) plays a critical role in regulating the inflammatory processes affecting the gingiva and the alveolar bone in periodontitis.5 Additionally, NF-κB is a heterodimer comprising the p65 and p50 proteins, which are associated with the inhibitor of NF-κB (IκB) existing in an inactive form inside the cytoplasm. Nuclear factor-kappa B is activated through the phosphorylation of IκB by IκB kinase (IKK) and causes the expression of several selected genes, including those for inflammatory cytokines, such as interleukin-6 (IL-6), IL-1β and tumor necrosis factor-alpha (TNF-α).6, 7, 8 Three subunits make up the IKK complex – IKKα, IKKβ and IKKγ – and each is encoded by a different gene.9
IKKβ is essential for activating NF-κB and producing cytokines in response to inflammatory and innate immune stimuli, and is associated with the stimulation of malignancies, such as breast cancer, pancreatic cancer, melanoma, and acute myeloid leukemia. Additionally, it is a crucial mediator of inflammation-induced bone loss (as in periodontitis), a regulator of bone homeostasis, and it is necessary for in vivo osteoclastogenesis. However, the primary role of IKKβ in osteoclastogenesis is to prevent TNF-α from inducing the death of osteoclast precursors.8, 9, 10
Polymorphisms, most commonly single-base variations or single-nucleotide polymorphisms (SNPs), may affect gene expression or the protein synthesis rate, and influence the periodontium through the immunological reaction, thus increasing susceptibility to periodontitis.11, 12 The examples of such SNPs are IKKβ at rs17875746 and rs12676482. However, no previous study has reported correlations between IKKβ genetic defects or variants and periodontitis. Furthermore, no study has evaluated the potential genetic association between IKKβ polymorphisms and periodontitis. Hence, this novel study hypothesized that IKKβ SNPs are associated with periodontitis in Iraqi Arabs. Additionally, the rationale of this study was that immunogenetics has the potential to provide novel and valuable knowledge regarding susceptibility to periodontitis.
Additional research is required to validate and broaden our basic knowledge on the genetic associations between periodontitis and inflammatory biomarkers that substantially impact the disease. These associations and biomarkers are expected to play a significant role in periodontitis.
Material and methods
Study design and setting
The study used a case–control design.
Blood samples were collected from subjects admitted to the Department of Periodontology of the College of Dentistry at the University of Baghdad, Iraq, and the Iraqi National Centre for Blood Donation/Ministry of Health from March to August 2022.
Participants
Eligibility criteria
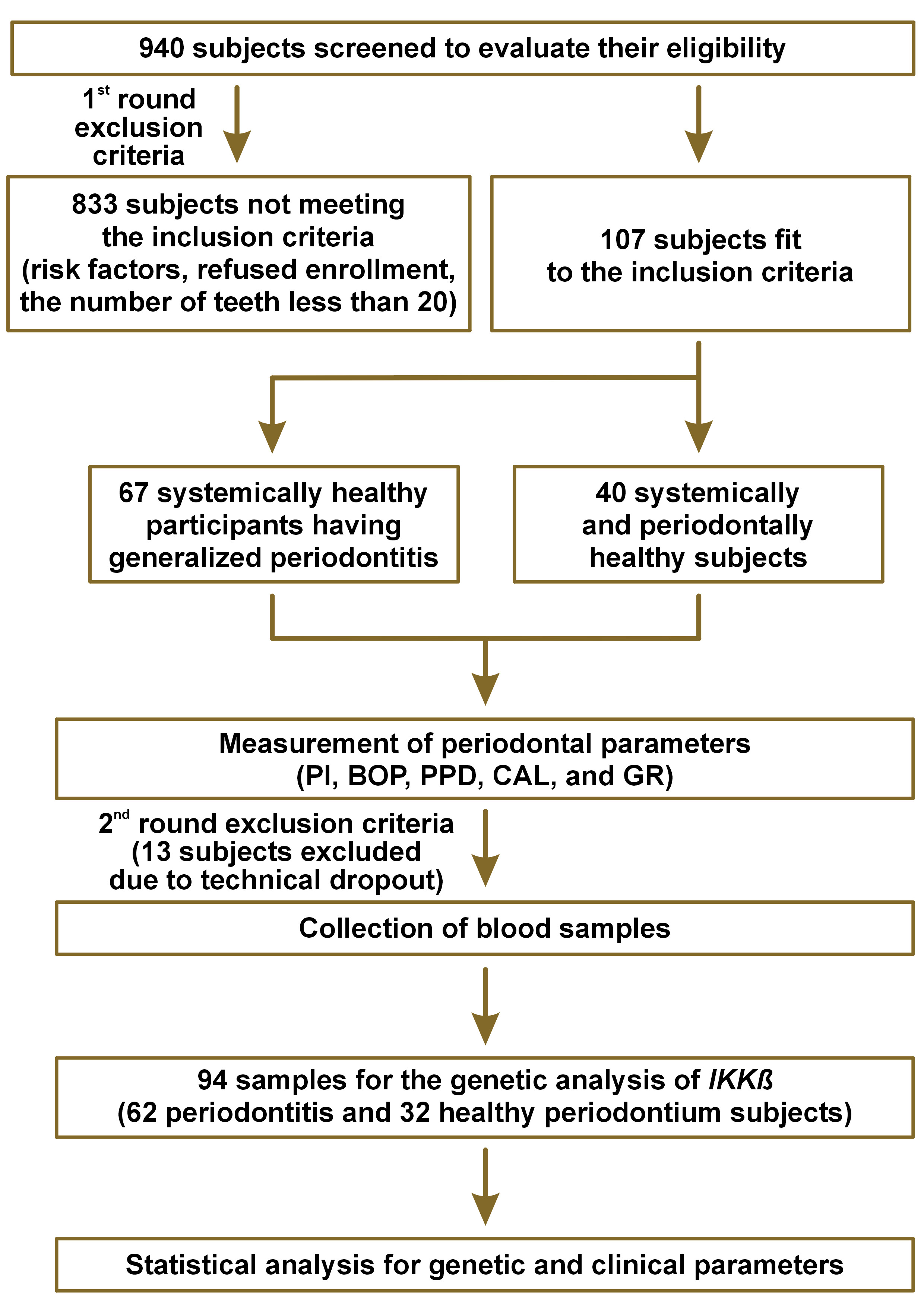
All participants (N = 94) were of the same ethnic background (Iraqi Arab), non-smokers, systemically healthy males and females, with ≥20 teeth. Any pregnant or lactating women, individuals with plaque retentive factors, such as orthodontic or prosthodontic appliances, and those receiving antibiotic therapy in the last 3 months or periodontal treatment in the previous 6 months were excluded (Figure 1).
Before blood specimens were collected, written informed consent was obtained from each patient. The Institutional Ethics Committee of the College of Dentistry approved the study (No. 453).
Allocation
The participants comprised 2 groups: the periodontally healthy control group (n = 32); and the periodontitis group (n = 62).
Variables
In particular, the criteria of the European Federation of Periodontology/American Academy of Periodontology (EFP/AAP) were used to diagnose periodontitis and determine which participants belonged to each group. Periodontitis must exhibit an unstable status, specifically, a probing pocket depth (PPD) ≥5 mm or PPD ≥ 4 mm with bleeding on probing (BOP), as well as the absence of the risk factors for periodontal disease (diabetes mellitus and smoking). The control group had the following characteristics: PPD ≤ 3 mm without clinical attachment loss (CAL); and BOP < 10%.13, 14
Measurements
Clinical parameters, including the plaque index (PI), BOP, PPD, CAL, and gingival recession (GR), and the number of mobile and missing teeth were measured using a manual Michigan 0 periodontal probe and recorded by the same examiner.
Around 2–3 mL of venous blood was drawn from each individual and transferred into an ethylenediaminetetraacetic acid (EDTA) tube (1.5 mg/mL) for the genetic analysis of IKKβ by means of the polymerase chain reaction (PCR) sequencing methods. These methods included DNA extraction, PCR amplification, direct Sanger sequencing, and data analysis.
Bias
Inter-examiner and intra-examiner calibration were conducted on 5 subjects until an agreement level of >0.75 was reached for all clinical parameters. The inter-examiner and intra-examiner consistency for continuous data (PPD, CAL and GR) was determined using an interclass correlation coefficient (ICC). Meanwhile, for categorical data (PI and BOP), the κ test was used to examine whether the correlations were significant. Any differences were discussed, and the sessions were repeated until the desired agreement level was reached.
Sample size
A pilot study was conducted using the first 12 samples collected from each group, following an allocation ratio of 1:2 for financial reasons (i.e., 4 periodontally healthy samples and 8 periodontitis samples). The samples were analyzed using the PCR sequencing methods to validate the primers and calculate the sample size. The sample size was calculated with the use of an online calculator – EpiTools (https://ausvet.com.au) – at a 95% confidence interval (CI) and a power of 80%, using the odds ratio (OR) of genetic polymorphisms of IKKβ at rs17875746 between the control and periodontitis groups. The OR was 5.5 in the pilot study and thus, the sample size required for the current study was 87 (29 for the control group and 58 for cases per the allocation ratio of 1:2). This number was increased to 94 subjects to account for dropouts during the laboratory tests. The values obtained from the pilot study were added to the final data of the main study.
Quantitative variables
The DNA was isolated from the samples by using an ABIOpure™ extraction kit (Alliance Bio, Bothell, USA). Then, the extracted DNA was quantified using a Quantus™ fluorometer (Promega Corporation, Fitchburg, USA). All DNA was stored at –40°C until tested. The primers were created by extracting the IKKβ gene sequence from the National Center for Biotechnology Information (NCBI) and using PrimerQuest (Integrated DNA Technologies, San Diego, USA), which covered most of the IKKβ gene, to find any potential SNPs present in the study groups and compare them to the expected sequences.
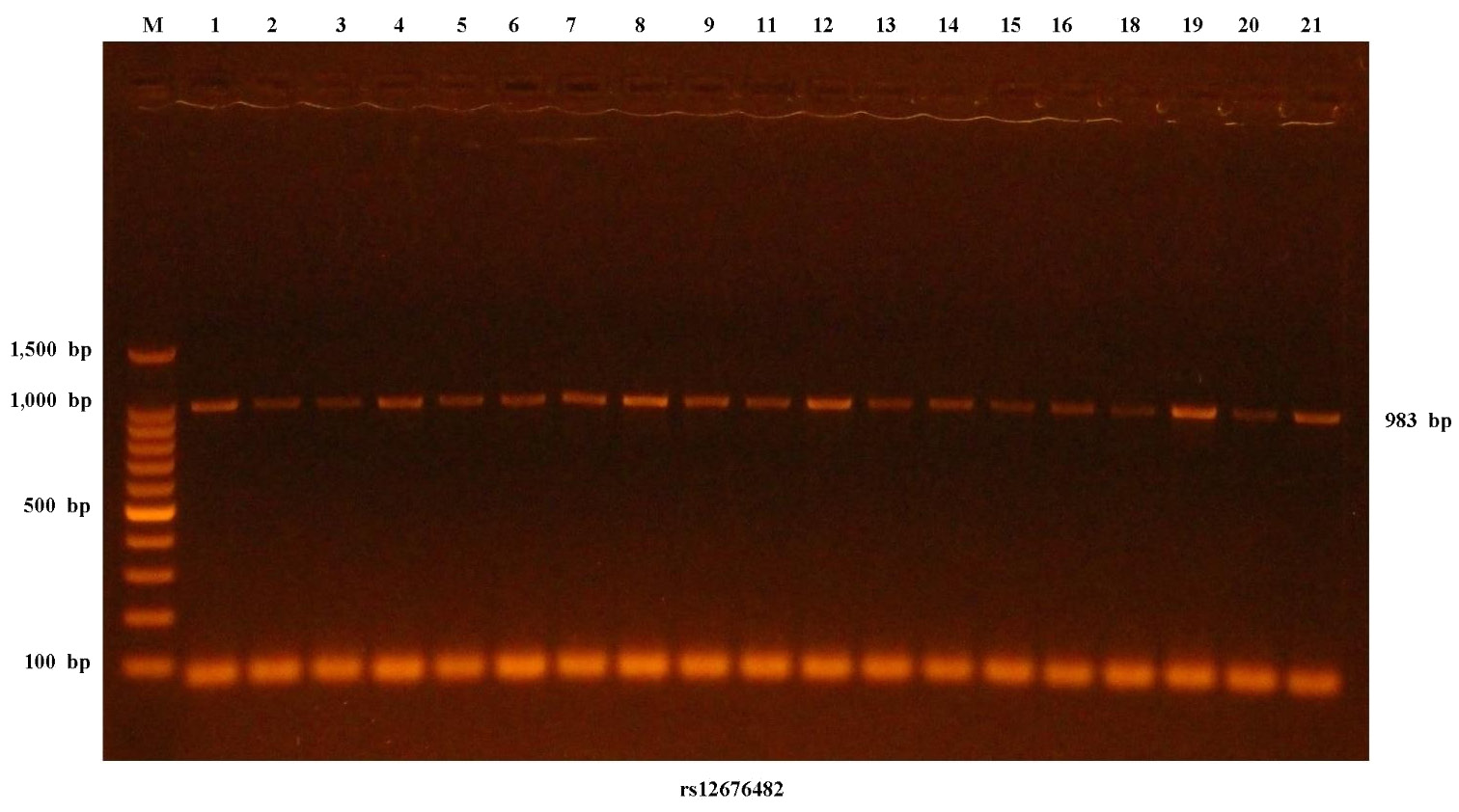
The DNA samples were amplified using forward and reverse primers (TGTAAAACGACGGCCAGTGCTCTCATGGGTCATTCTTATG and CAGGAAACAGCTATGACCACCTTGCCGTCTGAAATAC) in a lyophilized form with a product size of 983 bp (Macrogen, Seoul, South Korea). The primers were optimized at 55°C, 58°C, 60°C, 63°C, and 65°C to assess the ideal temperature and increase the accuracy of PCR amplification. The PCR amplification procedure for DNA was performed with a new, fast and ready-to-use PCR Express thermal cycler (Bio-Rad Laboratories, Hercules, USA). The procedure included 32 cycles, with 1 cycle at 94°C for 4 min (for initial denaturation), 30 cycles at 94°C for 30 s (for denaturation), at 60°C for 30 s (for annealing) and at 72°C for 30 s (for extension), and then 1 cycle at 72°C for 7 min (for final extension). The steps were stopped throughout incubation for 10 min at 4°C.
The PCR product of each sample (5 μL) was added to agarose gel, and electrophoresis was performed using an agarose gel kit (Promega Corporation) to verify the existence of PCR amplification for 1 h (at 100 V and 50 A). After electrophoresis, the separated DNA bands in the gel were visualized under ultraviolet (UV) light, and the images obtained by using gel imaging equipment (Major Science Co., Ltd., Taoyuan City, Taiwan) were saved for further analysis (Figure 2).
When gene fragments of the expected size appeared in PCR, the results were transmitted to Macrogen in South Korea, where the nucleotide sequences of these fragments were identified. The Basic Local Alignment Search Tool (BLAST), available from the NCBI site (http://blast.ncbi.nlm.nih.gov/Blast.cgi), was used to analyze the sequences.
Statistical analysis
All data collected in this study was analyzed using IBM SPSS Statistics for Windows, v. 28 (IBM Corp., Armonk, USA). The mean and standard deviation (M ±SD), median (Me), range, and number and percentage (n (%)) values are shown. The Mann–Whitney U test compared the 2 study groups, with the χ2 test used to compare categorical variables. Meanwhile, Spearman’s correlation coefficient (r) evaluated the association between polymorphisms and clinical variables.
The Hardy–Weinberg equilibrium (HWE) test was used to calculate the expected alleles from the observed genotypes. The association between a particular genotype and the disease risk was expressed with OR. Specifically, OR = 1 indicated no association, OR < 1 indicated a decreased risk and OR > 1 indicated an increased risk, while p-values <0.05 were considered significant.
Results
Descriptive data
Table 1 provides an overview of the findings and the distribution of the demographic and clinical variables of the groups. According to the Mann–Whitney U test, the periodontitis group had significantly greater PI and BOP than the control group, and significantly more mobile and missing teeth according to the χ2 test (p ≤ 0.001). Furthermore, the control group was free of PPD, CAL and GR.
Outcome data
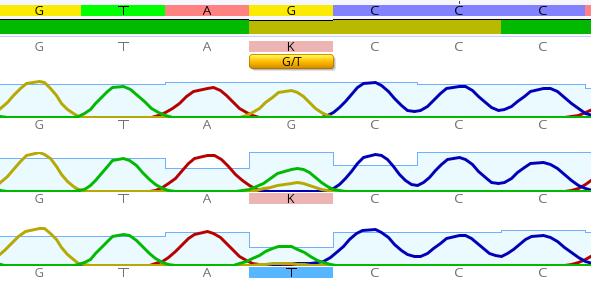
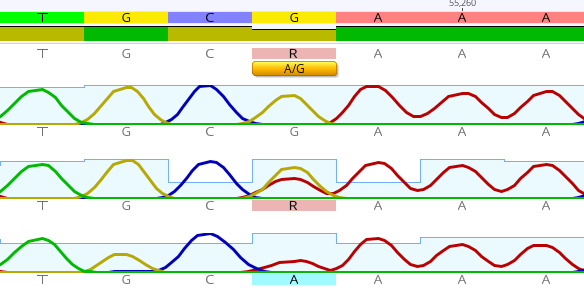
After sequencing with the Sanger method, 2 polymorphisms were detected in the IKKβ gene at rs17875746 and rs12676482. Moreover, there was a substitution of guanine (G) with thymine (T) in the rs17875746 SNP, while in the rs12676482 SNP there was a substitution of G with adenine (A), as shown in Figure 3 and Figure 4, respectively.
The HWE tests for rs17875746 for each group and the total sample revealed significant differences between the observed and expected SNPs in the periodontitis group and the total sample (p ≤ 0.001), while the results of the equilibrium for rs12676482 were non-significant, as shown in Table 2.
Main results
The SNP type, number and distribution for both groups are illustrated in Table 3. All SNPs detected after the sequencing of the IKKβ gene were of a single-base substitution, as there were 9 transversion SNPs in the periodontitis group and only one in the control group that belonged to rs17875746, exhibiting a non-significant difference. Meanwhile, there were 18 transition SNPs in the periodontitis group for rs12676482 and 10 in the control group for the same SNP, which represented a non-significant difference. The effect of the distribution of IKKβ SNPs on periodontitis was assessed based on OR with 95% CI, which was 5.264 for rs17875746 and 0.900 for rs12676482.
The genetic analysis of IKKβ SNPs revealed the allele frequency for both SNPs in the study groups (Table 4). For rs17875746, there was a significantly higher frequency of allele G in the healthy group than in the periodontitis group. For rs12676482, the frequency of allele G was higher in the periodontitis group (83.9%) than in the healthy group (79.7%), while the control group showed a higher frequency of allele A (20.3%) than the periodontitis group (16.1%). However, the differences between the groups for alleles G and A for rs12676482 were non-significant (p > 0.05). Surprisingly, the frequency of allele T had a high association with periodontitis (OR = 6.750) for rs17875746. Moreover, the χ2 test was conducted to determine the genotype frequency of all SNPs in IKKβ. The results showed non-significant differences in their distribution between the periodontitis and control groups (Table 4).
Other analyses
In a more detailed description, the results illustrate the impact of each genotype of IKKβ SNPs on the occurrence and prevention of the disease. The GT genotype in rs17875746 had a higher chance (OR = 3.321) for developing periodontitis than other genotypes. Meanwhile, the GA genotype in rs12676482 had a 1.242 chance of developing the disease, which was higher than for other genotypes.
The statistics presented in this study indicate a significant positive association between rs17875746 and tooth mobility, a family history, CAL (p = 0.034), and GR (p = 0.011) in the study groups, while the associations of rs17875746 with other parameters were non-significant (p ≥ 0.05). In addition, the associations of rs12676482 with all parameters were non-significant (p ≥ 0.05), as shown in Table 5. Furthermore, the results showed a non-significant correlation between the SNPs of IKKβ in the study groups, which is illustrated in Table 6.
Discussion
Key results
Periodontitis can be defined as a complex genetic infectious disease. Although periodontal pathogens are an initiating factor for periodontitis, other environmental and genetic risk factors play a critical role in its pathogenesis. Identifying these risk factors is crucial for the effective prevention and management of the disease,15, 16 which would influence the quality of life of adults in Iraq.17, 18 The significant differences in PI and BOP between the control and periodontitis groups shown in Table 1 can be explained by the fact that microbial plaque is the primary cause of periodontal disease.3, 15, 19 In previous research, Nascimento de Macêdo et al. reported that a PI of 65% or more than 4 missing teeth were positively associated with periodontal tissue destruction20; this result is consistent with the findings of the current study.
The induction of IKKβ by bacterial endotoxins, such as lipopolysaccharides, during the immune response activates interferon regulatory factors (IRFs) and NF-κB in tissues.8, 21 Nuclear factor-kappa B plays a critical role as a potent inflammatory mediator, and a stimulator of osteoclastogenesis and bone resorption during periodontitis. Thus, IKKβ is a crucial modulator of osteoclast preservation and a necessary component of inflammation-induced bone loss, unlike IKKα.10 This finding is corroborated by the significant positive association between IKKβ rs17875746 and tooth mobility, CAL and GR.
Interpretation
Variations in the IKKβ gene have been linked to many inflammatory and proliferative diseases through the secretion of inflammatory cytokines, such as IL-1β and IL-6, which have substantial effects also on the pathogenesis of periodontitis.8, 22 Thus, the gene polymorphisms of IKKβ may influence the genetic susceptibility profile of periodontitis by influencing the inflammatory response. A study in mice by Kure et al. suggested that periodontal disease patients might benefit from an effective prevention or suppression method that would inhibit IKK via the down-regulation of NF-κB.23
The most common type of genetic polymorphism is SNP. In this case, a single nucleotide at a specific point is substituted by another nucleotide, and the structure and function of the gene are altered, which may consequently result in different diseases, like periodontal diseases and dental caries, especially in obese patients and those with poor oral hygiene.24, 25, 26, 27, 28
The genetic variants of IKKβ polymorphisms at rs12676482 have been evaluated for their role in various disease states, such as the tumor risk in nasopharyngeal carcinoma tissues29 and the risk of systemic lupus erythematosus in the Chinese population.30 However, another study on the Chinese population showed no genetic predisposition to systemic lupus erythematosus risk at rs12676482.31 Owing to such inconsistent results, additional research is required to confirm the precise process.
Identifying genetic susceptibility to periodontitis could be very useful for developing individual treatment strategies, new diagnostic methods and more effective preventive measures, as well as for gaining a better understanding of periodontitis pathogenesis.19 Thus, in the present study, 2 SNPs of the IKKβ gene at rs17875746 and rs12676482 were investigated and tested to determine their associations with the periodontitis risk in a sample of the Iraqi population. Unfortunately, no references or studies were available to make comparisons with the current results on IKKβ polymorphisms.
Complex diseases such as periodontitis are characterized by polygenic causes, and the genes involved in these diseases are regarded as susceptibility genes.32 Many susceptibility genes are thought to be related to periodontitis, but the number and type of such genes in similar situations can vary among different forms of the disease and ethnicity.33 Therefore, in this study, we observed patients of the same ethnic background (Iraqi Arab), with no significant differences in age and sex, to avoid any bias.
The results show the distribution of the SNP genotypes in an Iraqi population. Homozygous TT and heterozygous GT at rs17875746, and heterozygous GA at rs12676482 were the most prevalent genotypes in periodontitis cases in comparison with healthy controls (Table 4). However, the differences were not significant. Allele T (at rs17875746) and allele G (at rs12676482) were more prevalent in the periodontitis group as compared to controls; they were also associated with a higher disease risk, as indicated by the elevated OR (Table 4).
Our study has the obvious contribution of being the first to assess the possible association between SNPs in the IKKβ gene and susceptibility to periodontitis in an Iraqi Arab population. Furthermore, the main findings of the study specified that IKKβ polymorphisms at rs17875746 may result in greater susceptibility to periodontitis, which was supported by the elevated OR, as well as a positive association between SNPs and increased tooth mobility, CAL and GR in the periodontitis group.
Limitations
Our study has some limitations, with the sample size and the genetic heterogeneity of periodontitis being 2 restrictions that prevent the generalization of the findings. Furthermore, we did not detect the corresponding levels of the IKKβ gene and the IKKβ protein expression in each group. In this regard, the findings of this study require further investigation.
Conclusions
The present study revealed that polymorphisms at rs17875746 and rs12676482 of the IKKβ gene may be associated with genetic disease susceptibility in an Iraqi Arab population. Furthermore, a positive association between rs17875746 and tooth mobility, a family history, CAL, and GR indicates the potential role of the SNP in increasing the severity of periodontitis. Identifying genetic markers for periodontitis susceptibility would allow people at risk to be identified quickly, and might eventually aid periodontitis treatment through individualized therapy. However, more research is needed to evaluate the contribution of IKKβ to periodontitis in addition to other risk factors, i.e., pathological, genetic and environmental ones, because of its complicated nature.
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of the College of Dentistry at the University of Baghdad, Iraq (No. 453). Written informed consent was obtained from each patient.
Data availability
The data for IKKβ SNPs at rs17875746 and rs12676482 are deposited at the NCBI GenBank database under accession numbers LC738785, LC738786, LC738787, LC738788, LC738789, and LC738790.
Consent for publication
Not applicable.