Abstract
Background. Temporomandibular disorders (TMD) pose a serious health problem that can have a negative effect on patients’ lives, impair work performance, and result in work absences and restrictions in daily activities.
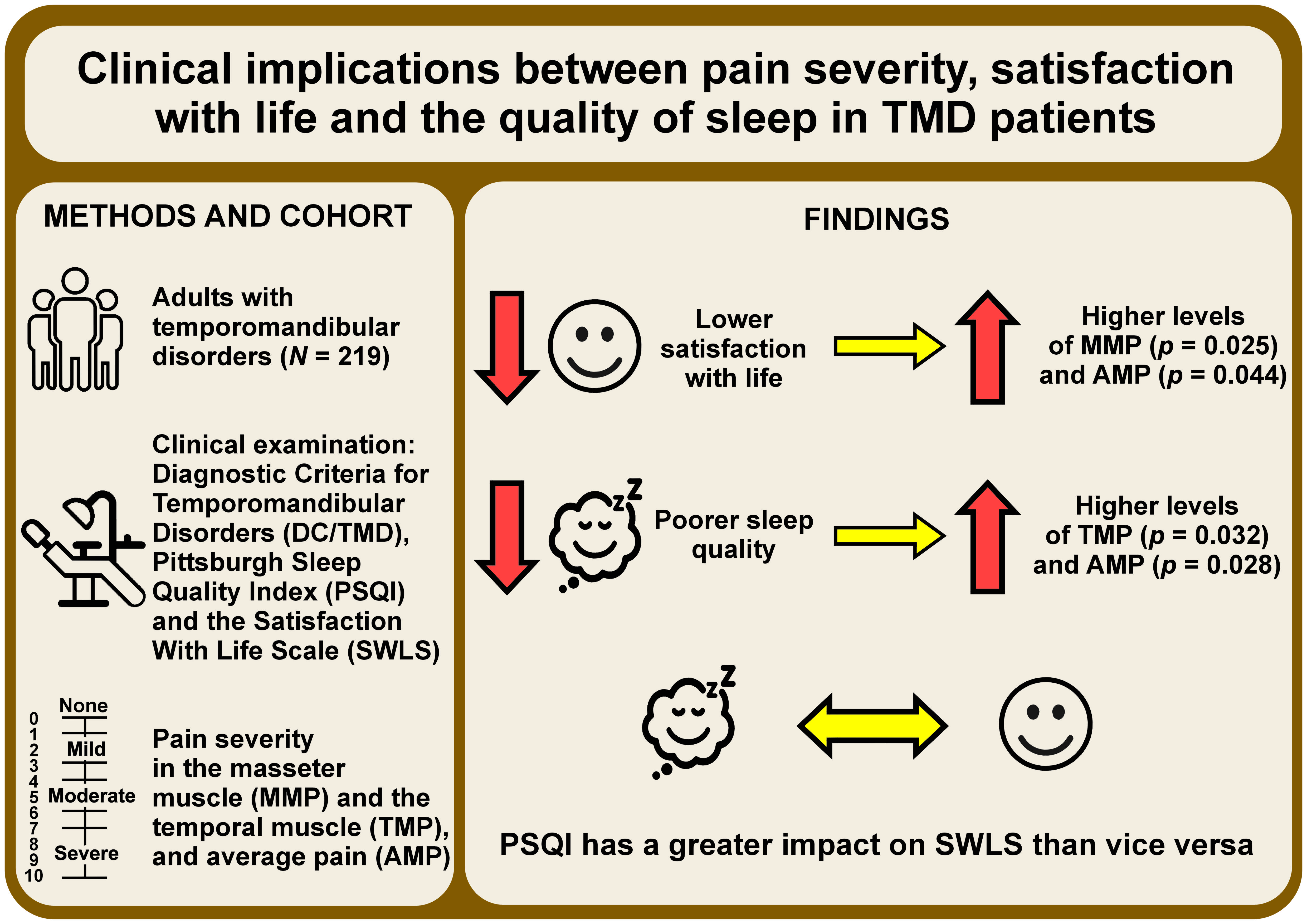
Objectives. The aim of this observational, cross-sectional study was to evaluate the level of satisfaction with life among Polish patients with TMD and to assess the influence of pain severity on this parameter. A secondary goal was to investigate sleep quality within this patient group and explore its relationship with pain.
Material and methods. A total of 219 patients from the Outpatient Clinic for Temporomandibular Disorders at the University Dental Polyclinic in Wroclaw, Poland, participated in this study. These individuals underwent a clinical examination using the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) protocol and completed 2 validated questionnaires, namely the Satisfaction With Life Scale (SWLS) and the Pittsburgh Sleep Quality Index (PSQI). Furthermore, the patients were assessed for the severity of masseter muscle pain (MMP) and temporal muscle pain (TMP), and the average pain in these muscles (AMP) was calculated. Subsequently, a statistical analysis was performed on the collected data.
Results. The group of patients with average satisfaction with life exhibited significantly higher levels of MMP (p = 0.025) and AMP (p = 0.044) as compared to the high-satisfaction group. Regarding sleep quality, 50.23% of the patients experienced poor sleep quality. Poor sleep quality was found to be statistically associated with higher levels of TMP (p = 0.032) and AMP (p = 0.028). Moreover, women demonstrated significantly worse sleep quality as compared to men (p = 0.002). The findings indicate that PSQI has a greater impact on SWLS than vice versa.
Conclusions. Due to a large number of TMD patients experiencing poor sleep quality and the associated reduced life satisfaction, these parameters should be considered as influential factors that modify the management of patients with TMD.
Keywords: satisfaction with life, sleep quality, temporomandibular disorders, orofacial pain, masticatory muscle pain
Introduction
“Temporomandibular disorders” (TMD) is an umbrella term that refers to a group of conditions involving the masticatory muscles, the temporomandibular joints (TMJs) and the associated structures.1 The typical manifestations of TMD include masticatory muscle pain or TMJ pain, limited jaw movement, joint sounds, such as clicking or crepitus, myofascial pain, and headaches.2 Temporomandibular disorders are a pervasive issue with significant implications for the quality of life, characterized by chronic pain and restrictions in daily activities. Moreover, the presence of elevated levels of anxiety, perceived stress, and even depression, commonly associated with TMD, poses a serious health problem that can impair work performance and result in work absences.3 It is considered the 2nd most common cause of oral region pain, following odontalgic pain.4 Additionally, it ranks as the 2nd main cause of musculoskeletal pain after chronic low-back pain.5
The prevalence of TMD is estimated to be between 5% and 12% in the general population,6 with a higher incidence of up to 30% among young adults.7 In the Polish population, approx. 55.9% of individuals experience at least one symptom of TMD.8 Gender differences are apparent, with women more commonly affected by TMD in the Polish population.8, 9, 10
The etiology of TMD is multifactorial and often unclear.2 The current biopsychosocial model encompasses various conditions and factors that may contribute to the occurrence of TMD.9 It incorporates psychosocial aspects, such as stress reactions, environmental factors, emotions, and cognition, with biological components, including structural and functional abnormalities.1 Numerous studies have demonstrated an association between TMD and psychological distress.11, 12, 13, 14, 15 A recent study conducted on a Polish population of TMD patients revealed a high prevalence of stress, anxiety and depression within the study group.13 Other studies indicate that these psychological factors can be risk factors for the development of TMD.14 Chronic TMD are also known to coexist with psychosocial impairments, such as depression and somatization.16 Importantly, some TMD patients may exhibit reduced treatment responsiveness due to their mental state. Studies suggest that patients with high levels of catastrophizing thinking are less likely to respond to treatment unless psychotherapy is utilized.17 The same applies to patients with depression and anxiety.13, 18 As a result, TMD patients are at risk of experiencing a significant reduction in their quality of life and social functioning.3, 15
Many studies indicate that TMD may have a negative effect on patients’ lives, manifested as chronic pain, the loss of energy, activity restriction (inability) due to physical ailments, emotional disorders, anxiety/depression, taste changes, discomfort when eating, and absence from work due to chronic pain.3 Consequently, the present study aimed to assess the level of satisfaction with life in Polish patients with TMD and to investigate the influence of TMD-related pain on this parameter. Additionally, since there is evidence suggesting an association between TMD and sleep quality,19, 20, 21, 22, 23 our secondary goal was to assess the relationship between sleep quality and masticatory muscle pain in Polish patients with TMD. The reason for undertaking this research is the lack of studies pertaining to the adult Polish population with TMD, using reliable methodological tools. The existing number of studies conducted with rigorous methodologies is limited, thus necessitating high-quality research.
Material and methods
Participants and the study design
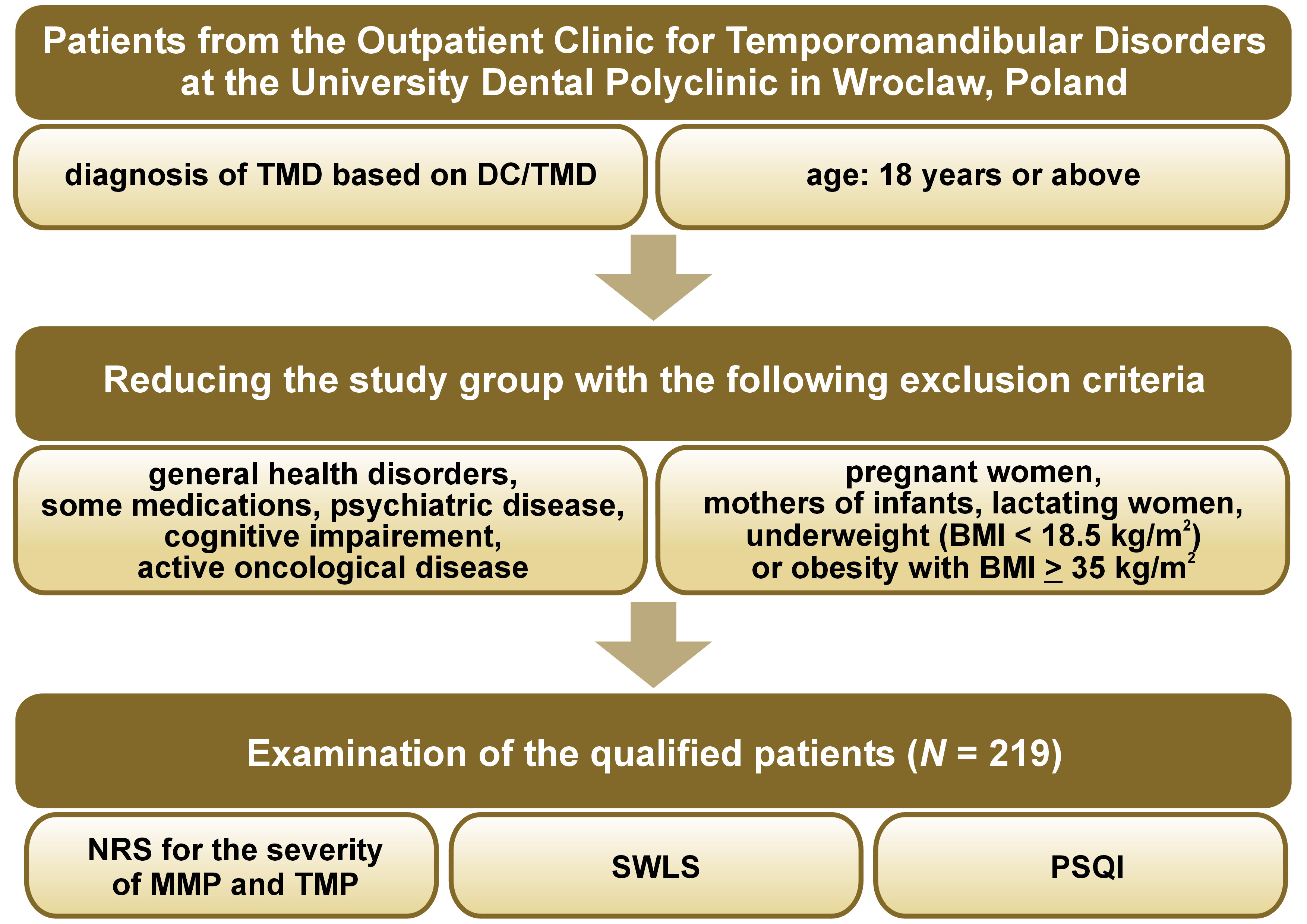
The study group comprised 219 adults who sought treatment at the Outpatient Clinic for Temporomandibular Disorders at the University Dental Polyclinic in Wroclaw, Poland. The study adhered to the principles outlined in the Declaration of Helsinki, and the research protocol obtained approval from the Bioethical Committee of Wroclaw Medical University (No. KB-165/2021). Furthermore, this observational, cross-sectional study was retrospectively registered in the ClinicalTrials.gov database on January 6, 2022, and received the registration number NCT 05183503.
Inclusion and exclusion criteria
The following inclusion criteria were established for the study group: a diagnosis of TMD based on the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) examination24; and an age of 18 years or older.
The exclusion criteria for the study were as follows:
– neurological conditions, such as cerebrovascular and neurodegenerative diseases, post-toxic changes, and inflammatory changes; systemic diseases, including rheumatoid arthritis, systemic lupus erythematosus and primary Sjögren’s syndrome; toxic or traumatic diseases related to head or craniofacial trauma;
– chronic use of medications that can affect neuromuscular function or the activity of the central nervous system (CNS), such as neuroleptics, analgesics, antiepileptic drugs, psychostimulants, steroids, sedatives, and hypnotics;
– history of psychiatric diseases, such as anxiety, depression, attention deficit hyperactivity disorder (ADHD), autism, and schizophrenia, as well as sleep disorders;
– cognitive impairment;
– active oncological disease;
– underweight (body mass index (BMI) <18.5 kg/m2) or obesity with BMI ≥ 35 kg/m2; and
– pregnant women, mothers of infants, and lactating women.
Data collection
Data was collected from November 2018 to December 2020; during that period, the patients were requested to complete questionnaires. Following the completion of the questionnaires, clinical examinations of TMJs and the masticatory muscles were conducted. The questionnaires administered to the patients included the Satisfaction With Life Scale (SWLS) and the Pittsburgh Sleep Quality Index (PSQI). Detailed descriptions of these questionnaires are provided later in the text. A dentist with a minimum of 5 years of practice and trained in the DC/TMD protocol conducted the clinical examinations. The patient diagnoses were assigned separately for each side, and multiple diagnoses were possible for each side.24
Clinical examination of the masticatory muscles and the temporomandibular joints (DC/TMD)
The DC/TMD protocol is a standardized tool utilized worldwide for assessing disorders related to the masticatory muscles and TMJs. It consists of 2 axes – Axis I focuses on diagnosing biological factors through physical examinations, while Axis II involves the assessment of psychosocial factors by using validated questionnaires. The protocol structure allows a comprehensive evaluation of patients, aligning with the biopsychosocial model.
Following the completion of the questionnaires, the participants underwent evaluation by experienced dentists with a minimum of 5 years of practice. All the examiners performing the assessments underwent training and calibration according to the protocol provided on the official website of the International Network for Orofacial Pain and Related Disorders Methodology (https://ubwp.buffalo.edu/rdc-tmdinternational). The training was conducted by a clinician with 10 years of experience in managing TMD. The diagnoses were assigned independently for each side, adhering to the official DC/TMD taxonomy.24, 25
Assessment of the masticatory muscle pain severity
During the clinical examination, each patient was requested to provide a rating for the perceived pain experienced during the palpation of the masseter and temporal muscles on each side individually. Subsequently, the average of these ratings was calculated. The numeric rating scale (NRS), a widely utilized and well-validated tool for measuring pain intensity, was employed for this purpose. The scale ranges from 0 to 10, with 0 indicating no pain and 10 representing the worst imaginable pain.26 In his study, the severity of masseter muscle pain (MMP) and temporal muscle pain (TMP), as well as the average muscle pain (AMP) were calculated.
Questionnaires
Satisfaction With Life Scale
This questionnaire serves as a simple yet reliable instrument for assessing life attitudes. It consists of 5 questions, and patients assign a score ranging from 1 to 7 for each question. A score of 1 signifies ‘strongly disagree’, while a score of 7 represents ‘strongly agree’. The total score is obtained by summing the scores for all questions. The lowest possible score is 5, while the maximum score is 35. In this questionnaire, the scores were categorized into STEN groups: STEN 1–4 (5–17 points) indicates low life satisfaction; STEN 5–6 (18–23 points) indicates average life satisfaction; and STEN 7–10 (24–35 points) indicates high life satisfaction.27
Pittsburgh Sleep Quality Index
This questionnaire serves as a self-report tool for assessing sleep quality over a 1-month interval. It comprises 19 items creating 7 components that measure various aspects of sleep, including subjective sleep quality, sleep latency, sleep disturbances, and sleep duration, among others. Each component is rated on a scale from 0 to 3. The scores for all components are summed, resulting in a maximum score of 21. A cutoff point of 5 was established, where higher scores indicated poorer sleep quality. The questionnaire used has demonstrated reliability as a tool for sleep assessment.28
Sample power and size calculation
The sample size was calculated using G*Power, v. 3.1.9.7 (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower). The determination aimed for a statistical power of 0.8, an effect size of 0.22 for correlations between SWLS and NRS AMP and an effect size of 0.40 for correlations between PSQI and NRS AMP. It was ascertained that the sample size needed was 207 people for the 1st dependency and 208 people for the 2nd one. Therefore, 219 patients were qualified for the study to be able to detect significant differences and correlations between the studied parameters. The process of recruiting patients for the study is presented in Figure 1.
Statistical analysis
Statistical analysis was conducted using the Statistica software, v. 13 (StatSoft Polska, Cracow, Poland), with a significance level set at 0.05. The distribution of data was assessed using the Shapiro–Wilk test. Categorical data were presented as number and percentage (n (%)). The NRS values for the masseter and temporal muscles were computed as the average of the values obtained from the right and left sides. Consequently, the relationship between the NRS values and the TMD diagnosis was examined using the Mann–Whitney U test. The same approach was employed to analyze the association between the pain levels and gender.
Results
Sample characteristics
The study involved a sample of 219 Caucasian Polish adults diagnosed with TMD. Among the participants, 163 (74%) were women and 56 (26%) were men. The age range of the participants was 18–80 years, with a mean age of 40.06 ±16.37 years. Most of the group was comprised of women, with a female-to-male ratio of 3:1. Statistically significant differences were observed between women and men in terms of age. The mean age for women was 42 ±16.95 years, while the mean age for men was 34 ±13.17 years.
Distribution of temporomandibular disorders
The distribution of diagnoses among the participants was as follows: myalgia was the most common diagnosis, being predominant in both women and men; the 2nd most frequent diagnosis was disk displacement with reduction, which was also dominant in both genders; and the 3rd most common disorder was arthralgia. It is worth noting that a single patient could have multiple DC/TMD diagnoses. More specific information regarding the distribution of diagnoses by gender can be found in Table 1.
In terms of correlation between the DC/TMD diagnosis and the age of the patients, no statistically significant relationships were observed (p > 0.05).
Pain intensity (NRS)
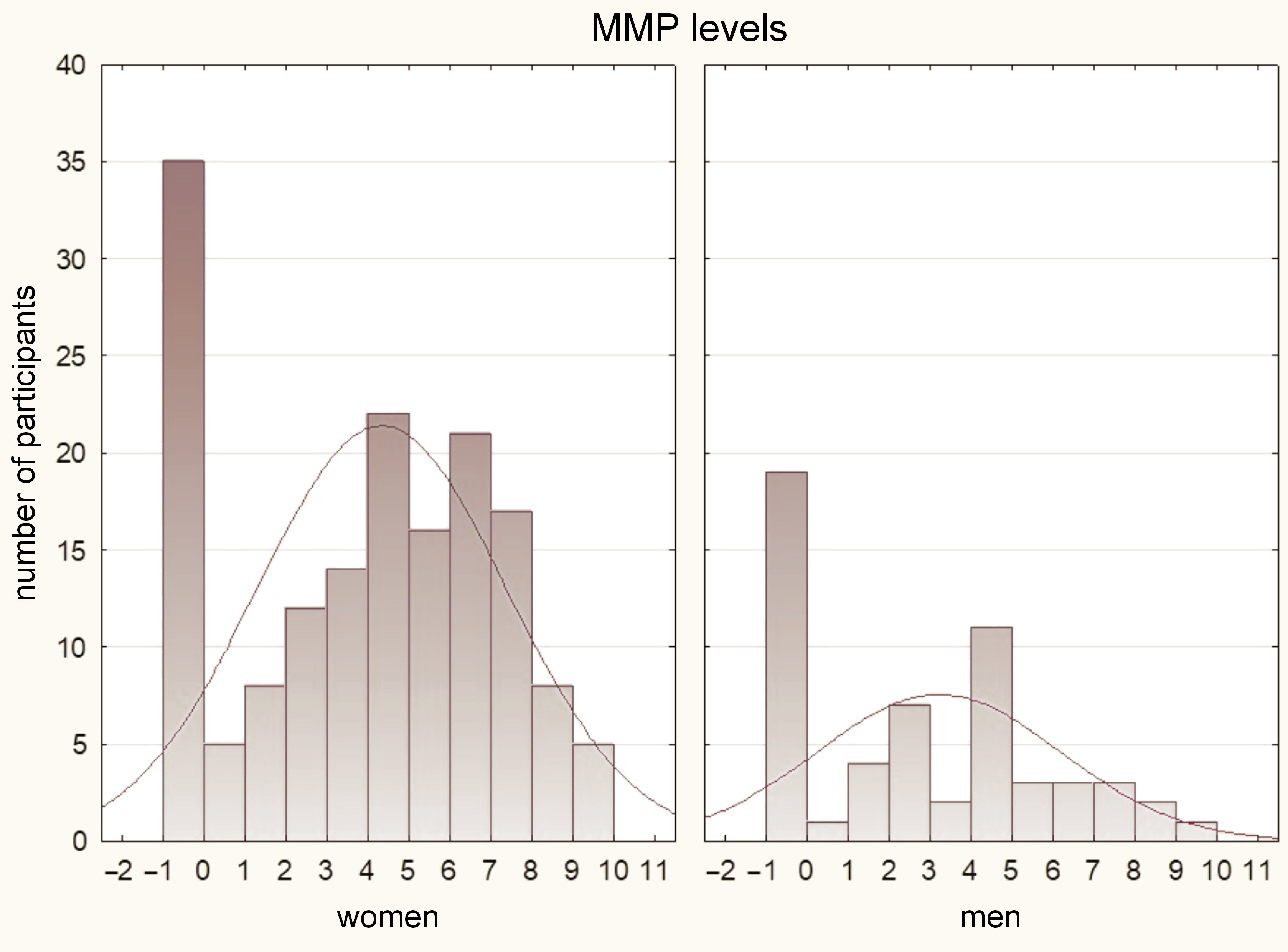
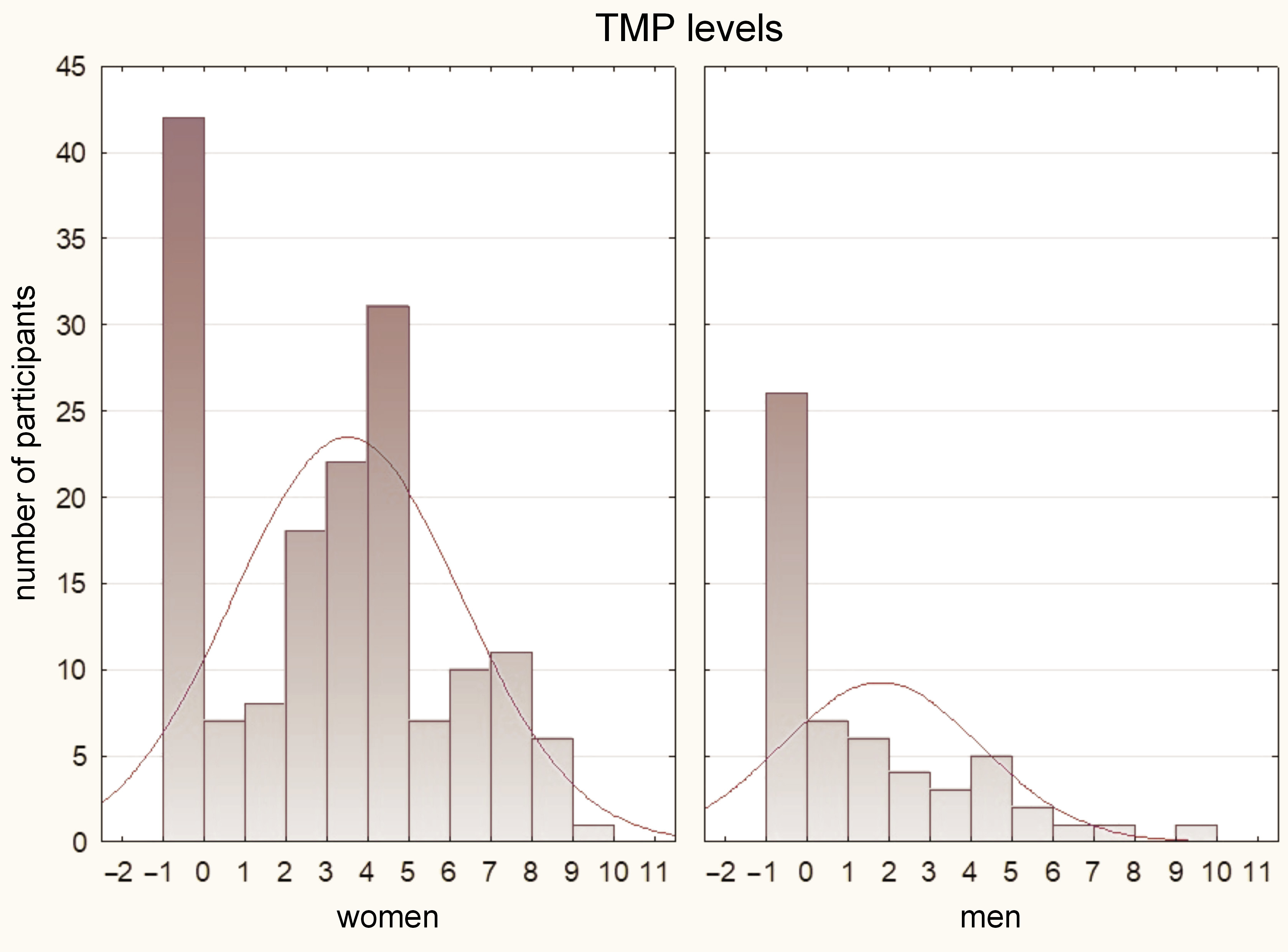
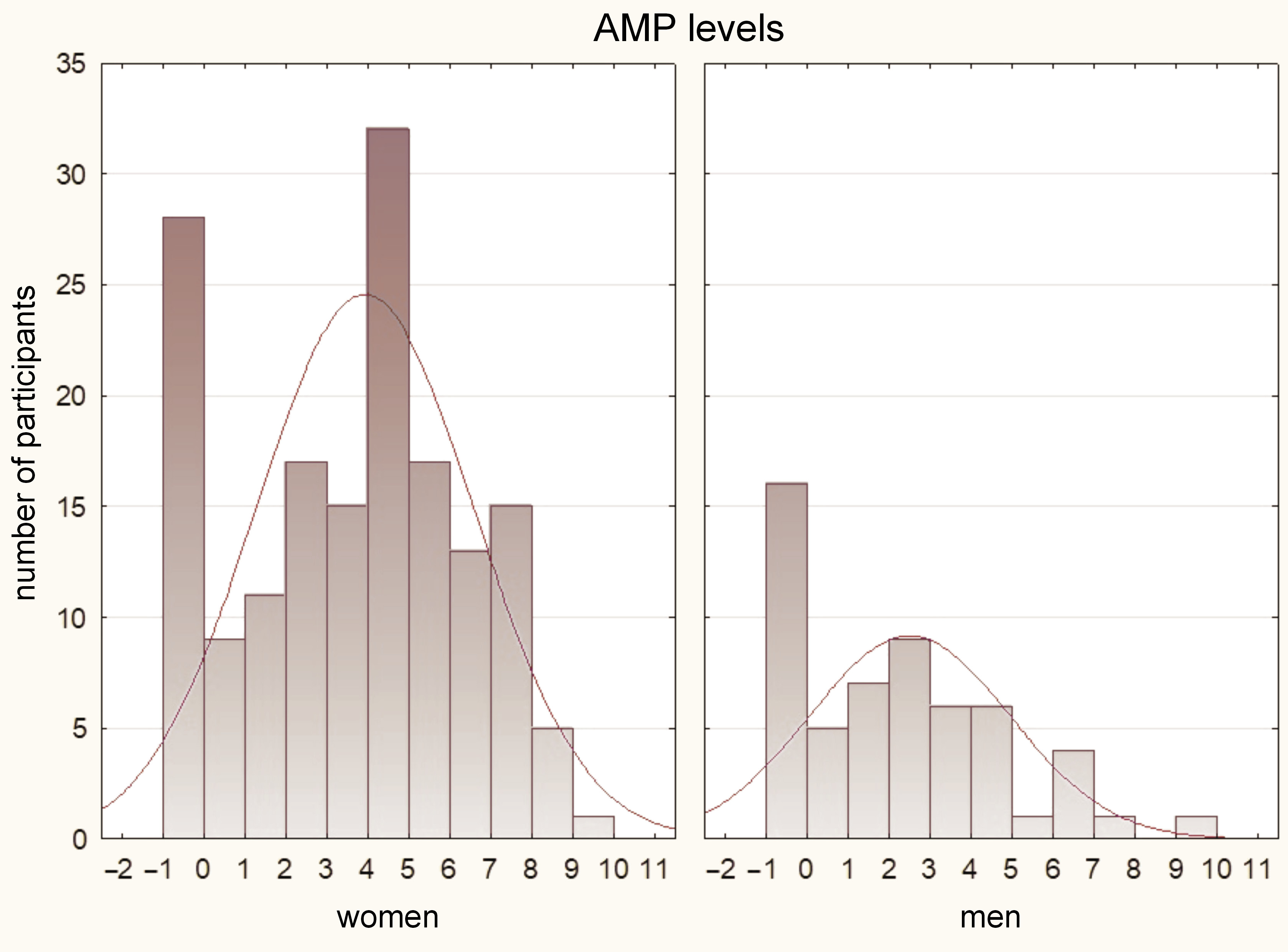
The results of the Mann–Whitney U test indicated that women had significantly higher pain values as compared to men in all assessed parameters (p < 0.05). These findings are illustrated in Figure 2, Figure 3, Figure 4.
Regarding the analysis of the relationship between age and pain, the Kendall τ test was conducted, revealing no significant correlation between these 2 variables (p > 0.05).
In terms of DC/TMD diagnosis, several significant results were obtained. The Mann–Whitney U test demonstrated significantly higher levels of pain in the diagnosis of myalgia for MMP, TMP and AMP. For the diagnosis of myofascial pain, significantly higher pain values were observed for TMP and AMP. In the case of disk displacement without reduction, significantly higher pain results were found for MMP and AMP. However, there were no significant differences in the pain values among the other diagnoses (p > 0.05). Detailed results of the statistical analysis can be found in Table 2.
Satisfaction With Life Scale
In this questionnaire, 60% of the participants (n = 132) achieved STEN 7–10 indicating high life satisfaction. In 28% of the participants (n = 61), STEN 5–6 was obtained, representing average satisfaction with life, while only 12% of the participants (n = 26) achieved STEN 1–4 indicating low life satisfaction.
To investigate the relationship between the SWLS scores and gender, Somers’ D test was performed, revealing no significant correlation between these variables (d(X|Y) = 0.12664). Furthermore, the Kruskal–Wallis analysis of variance (ANOVA) was conducted and showed no significant differences between the STEN groups (p = 0.529).
To assess the correlation between the specific TMD diagnoses and the SWLS scores, Somers’ D test was conducted. This test, being asymmetrical, enables the analysis of the influence of the TMD diagnosis on the SWLS score and vice versa. However, no dependencies were found in either direction. None of the results exceeded the cutoff value of 0.4, which was considered significant.
The statistical analysis regarding pain was performed using the Kruskal–Wallis ANOVA. This analysis revealed a significant difference between the STEN 5–6 and STEN 7–10 groups in terms of MMP (p = 0.025) and AMP (p = 0.044). In both cases, the STEN 5–6 group exhibited higher pain values. However, no significant differences were observed between the STEN 1–4 group and the other groups (p > 0.05). It is worth noting that the lack of significance may be attributed to the small number of participants in this section (n = 26).
Pittsburgh Sleep Quality Index
Out of the study group, 217 participants completed the questionnaire correctly. Regarding the cutoff point, the participants were almost evenly split. A score below 5 was obtained by 108 patients (49.77%), while a score above 5 was acquired by 109 patients (50.23%). This indicates that over half of TMD patients experience poor sleep quality.
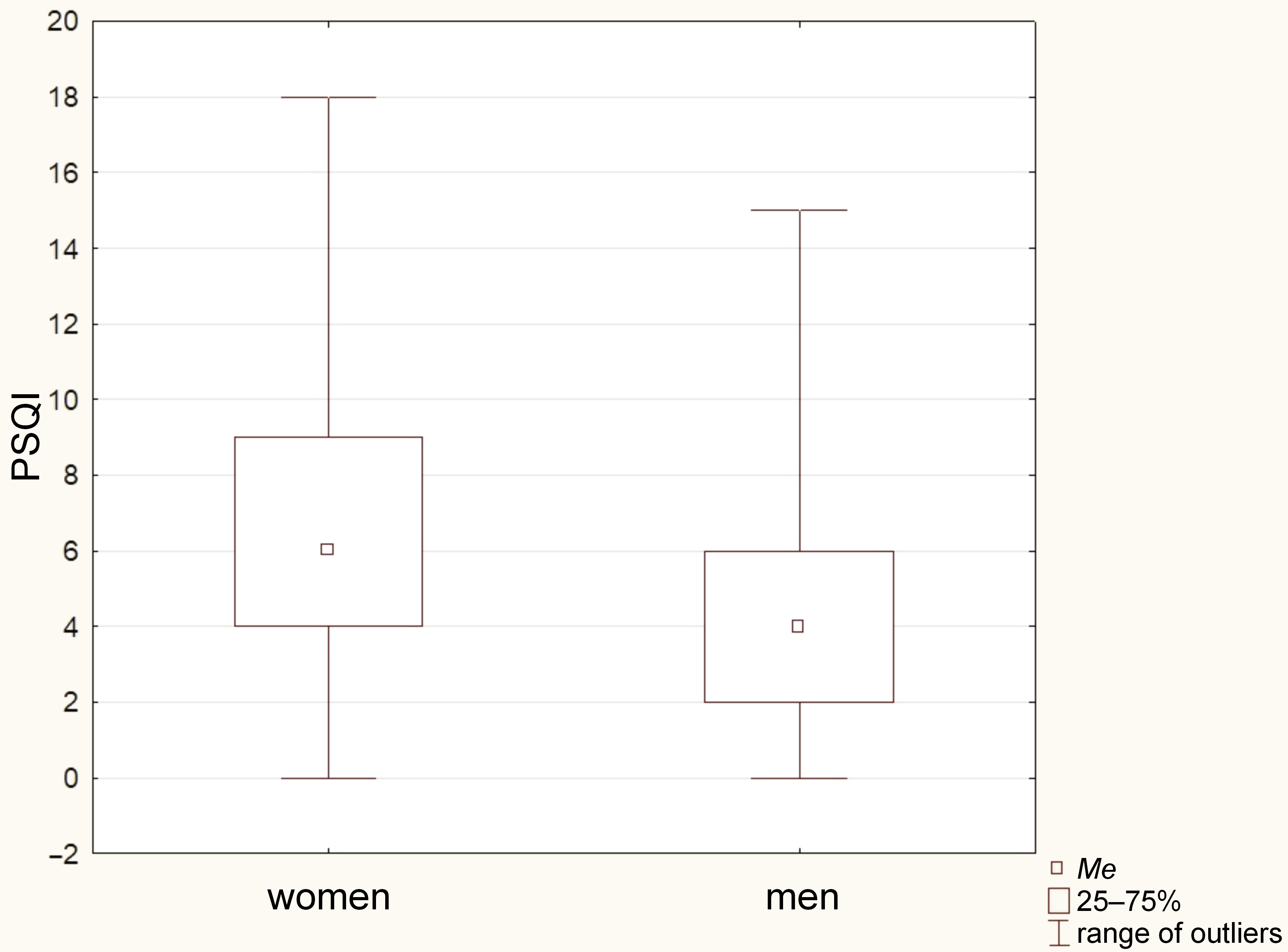
To analyze the relationship between age and the PSQI results, the Mann–Whitney U test was employed. However, no significant differences between the groups was found (p = 0.226). In terms of gender, the χ2 test was conducted, revealing that women scored significantly higher in PSQI as compared to men (p = 0.002). Figure 5 illustrates this difference, using a box-whisker chart.
To assess the relationship between the specific TMD diagnoses and the PSQI scores, the χ2 test was employed. However, no significant correlation was found for any of the TMD diagnoses (p > 0.05).
In terms of relationship between pain and the PSQI scores, the Mann–Whitney U test was conducted. The results demonstrated that participants with a PSQI score above 5, indicating worse sleep quality, exhibited higher levels of TMP (p = 0.032) and AMP (p = 0.028). Although not statistically significant, there was a close approach to significance for MMP (p = 0.059).
Satisfaction With Life Scale vs. Pittsburgh Sleep Quality Index
To examine the relationship between PSQI and SWLS in the study group, Somers’ D test was conducted. The analysis revealed a significant correlation between these 2 variables. The relationship was negative and moderate (X|Y = −0.4150), indicating that worse sleep quality was associated with lower scores on SWLS. As Somers’ D test is asymmetrical, it was possible to determine the reverse relationship as well. However, a lesser correlation was obtained (Y|X = −0.3807), which did not exceed the level of significance. These results suggest that the impact of PSQI, representing the quality of sleep reported by the patient, on the level of life satisfaction is greater than the reverse relationship.
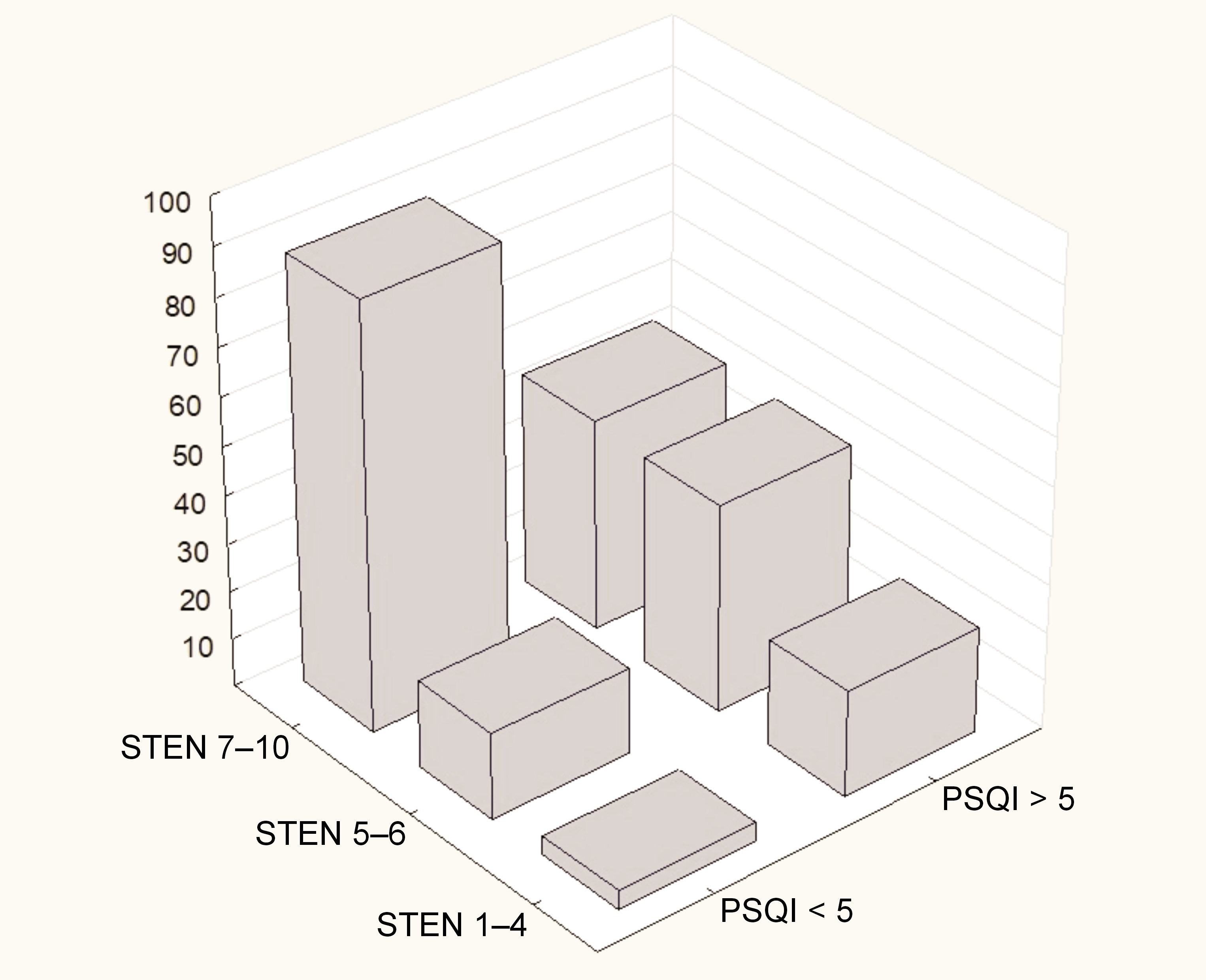
These findings confirm the earlier conclusions drawn from the relationships between the pain levels, sleep quality and satisfaction with life. It can be concluded that the level of satisfaction with life in TMD patients is influenced by various factors, including the experienced pain levels and factors such as sleep quality. This is particularly important, as it explains why the level of pain experienced is not always the sole determinant of the treatment response. Other accompanying symptoms, such as sleep disorders, stress, anxiety, and depression, may play a role in the patient’s response to therapeutic interventions. Therefore, additional approaches, such as psychotherapy, may be beneficial for patients in this group. The distribution of the SWLS levels with regard to PSQI is presented in Figure 6.
Discussion
This study aimed to investigate the level of satisfaction with life and sleep quality in adult patients with TMD. The clinical examination of the patients was based on the standardized DC/TMD protocol, which is considered the gold standard in TMD assessment. To measure life satisfaction and sleep quality, self-report questionnaires were utilized, namely SWLS and PSQI. These questionnaires have been widely and successfully used in previous studies.19, 29, 30
In this study group comprising TMD patients, most of the participants were female, which aligns with findings from other studies on TMD.31, 32 Statistical analysis revealed that women reported significantly higher pain levels as compared to men. This observation may be attributed to the fact that myalgia, which is the most common type of TMD pain, is also more prevalent in women than in men.8 Notably, myalgia was also the most frequently diagnosed condition in this study group. Previous research by Tuuliainen et al. emphasized the association between muscle pain and psychological distress.33 Studies have shown that women are more likely to have higher levels of depression and anxiety, placing them at a higher risk for muscle-related TMD.13, 34, 35 Hormone fluctuations, biological differences and greater pain sensitivity in women may contribute to the higher prevalence of various TMD types among females as compared to males.6, 36
It is indeed interesting that despite myalgia being the most prevalent pain-related TMD type in this study, no correlation with satisfaction of life was observed. This finding contrasts with a different study, which indicated a significantly higher presence of myalgia in patients with depression.13 Although different questionnaires are used to measure these parameters, depression is generally associated with lower satisfaction with life. Another study by Boggero et al. observed significantly lower satisfaction with life in patients with myogenic TMD in Kentucky, USA.19 Interestingly, including the pain levels in the analysis weakened, but did not eliminate the effect, suggesting that a decrease in satisfaction with life in TMD patients may be connected not only to the pain levels.
On the other hand, in the present study, the levels of pain in the masticatory muscles were significantly lower in the STEN 7–10 group as compared to the STEN 5–6 group, indicating that the intensity of painful sensations in these structures influenced satisfaction with life. This suggests that the diagnosis itself may not have as much impact on the quality of life as the level of pain experienced. The STEN 1–4 group did not show a significant difference as compared to the STEN 7–10 group, but as mentioned earlier, this group had only 26 participants. Since the methodology used in the abovementioned studies was similar, the observed differences may be attributed to regional and sociological factors within the study populations.13, 19
The question arises as to why the relationship between the TMD diagnosis and satisfaction with life is not as straightforward as expected. Based on our research, it can be concluded that the level of satisfaction with life in TMD patients is influenced not only by the presence of a specific diagnosis and the intensity of pain experienced, but also by other factors, such as anxiety, depression, stress, and sleep quality. The previously cited studies lead to similar conclusions.13, 19
Regarding sleep quality, the present study demonstrated that more than half of the participants experienced poor sleep quality. These findings are consistent with previous research on this topic.37, 38, 39, 40, 41, 42 Benoliel et al. observed that 43.3% of TMD patients reported poorer sleep quality as compared to 28.3% in the control group.37 Additionally, Sanders et al. found that participants with poor sleep quality had approximately twice the incidence rate of TMD as compared to those with good sleep quality in their cohort study.38
The conclusion drawn from this study aligns with the results of previous research investigating sleep quality in TMD patients.37, 38, 39, 40, 41, 42, 43 Sleep quality is an essential factor that, alongside pain and the psycho-emotional state of TMD patients, influences the reported complaints and modifies the treatment approach. This finding is of great importance for healthcare practitioners who treat TMD patients, as they should consider various comorbidities, including sleep disturbances.
Regarding specific TMD types, some authors have reported that patients with myofascial pain have poorer sleep quality as compared to patients with TMJ pain and controls.44, 45 In this study, no significant correlation was found between the specific TMD diagnoses and sleep quality. However, the group of patients experiencing more severe pain, particularly in the temporal muscle, demonstrated poorer quality of sleep. These results are consistent with the findings of Benoliel et al.37 A conclusion that can be drawn from these findings is that the level of pain is the most significant factor influencing sleep quality, rather than the specific TMD diagnosis. Differences in outcomes may arise from the fact that most participants in this study were diagnosed with myalgia, which differs from myofascial pain (which Lei et al. focused on in their study44) in terms of pain radiation patterns.24
Yap et al. concluded that, in general, patients with pain-related TMD experienced decreased sleep quality.29 Specifically, in their study, patients with myalgia demonstrated greater sleep impairment as compared to those with intra-articular disorders.29 Other studies have also suggested that musculoskeletal pain, not only in the temporomandibular region, is associated with poorer sleep quality, and this association is complex and influenced by various factors.41, 46 Conversely, poorer sleep quality can provoke and exacerbate head and orofacial pain syndromes, as sleep deprivation is known to affect pain perception.47 Psychological distress, including depression and anxiety, is considered a factor that influences sleep quality.39
Interestingly, in our previous research, we found that 54.8% of TMD patients experienced depression.13 This is a similar percentage to the number of patients with poor sleep quality in the present study, indicating a potential link between depression, sleep disturbances and TMD. The role of depression as a contributing factor in sleep disturbances was also suggested by Dubrovsky et al.42
The results of the present study indicate that women are more likely to experience poorer sleep quality as compared to men. This finding is consistent with previous studies on TMD patients, which have also observed a higher prevalence of depressive symptoms in women as compared to men. Given that depression can affect both TMD and sleep quality, these results contribute to a comprehensive understanding of the interconnections between these factors.
The relationship between TMD and sleep quality is indeed complex and influenced by multiple factors, including muscle pain and the psycho-emotional state of individuals. A study by Machado de Resende et al. showed that the TMD management involving occlusal splints, physical therapy and counseling could lead to improvement in sleep quality and the overall quality of life, supporting the notion that addressing TMD-related factors can have a positive effect on sleep.40
These findings contribute to the growing body of literature highlighting the prevalence of poor sleep quality as a common symptom in TMD, emphasizing the importance of sleep assessment in the diagnostic process of TMD.29, 30, 43
In this study, a significant correlation between sleep quality and satisfaction with life was observed. This finding aligns with previous research indicating an interaction between these 2 parameters. A study by Steptoe et al. demonstrated that individuals with poorer sleep quality reported lower levels of life satisfaction and experienced more negative emotions daily, including emotional stress and anxiety.46 In this study, we observed that sleep quality had a more significant impact on the perceived satisfaction with life than the other way round. However, it is important to note that only 12% of the patients in the study described their level of satisfaction with life as low. Most patients in the study group reported high (60%) or moderate (28%) life satisfaction.
Therefore, we can conclude that while pain is one component that affects the perceived satisfaction with life, it is not the sole factor. Patients with moderate satisfaction with life may experience higher levels of MMP as compared to those with high satisfaction with life. However, life satisfaction is also influenced by other factors, such as the levels of the perceived stress, anxiety and depression. Hence, it is crucial to consider these additional modulating factors and implement a differentiated approach to TMD management for patients with lower life satisfaction.
Limitations
The existing literature suggests a connection between muscle pain and psychological distress. Hence, questionnaires focusing on the psycho-emotional well-being of participants, such as the Patient Health Questionnaire-9 (PHQ-9) or the Generalized Anxiety Disorder-7 (GAD-7) questionnaire, could serve as valuable tools for addressing this matter. In this study, muscle pain was evaluated using NRS, which involved applying pressure to the muscles. To enhance precision and objectivity, the muscle pain threshold could be measured using an algometer. Additionally, the absence of a control group could be perceived as a limitation of the study. Another limitation of the study is the fact that no instrumental analysis of sleep was performed, and patients with sleep deprivation, e.g., shift workers with circadian rhythm disorders, were not excluded.
Conclusions
The conclusions drawn from our research indicate that screening for sleep quality and assessing satisfaction with life is a very important part of clinical evaluations with regard to Polish adults with TMD. Considering these factors in the diagnosis and management protocols can have a significant impact on pain intensity, pain-related disability and the patient’s response to TMD treatment. The questionnaires utilized in this study offer clinicians the means to identify patients with sleep disturbances or diminished life satisfaction, enabling specialized care and ensuring more effective assistance through multimodal management in this patient population.
Trial registration
The study was retrospectively registered in the ClinicalTrials.gov database on January 6, 2022, and received the registration number NCT 05183503.
Ethics approval and consent to participate
The research protocol obtained approval from the Bioethical Committee of Wroclaw Medical University, Poland (No. KB-165/2021). The patients provided their written informed consent to participate in the present study.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to privacy and ethical restrictions. However, they can be obtained from the corresponding author upon reasonable request.
Consent for publication
Not applicable.