Abstract
Background. The coronally advanced flap (CAF) technique is the preferred option in treating recession defects, especially when the attached gingiva is adequate. A laser-assisted vestibular releasing incision after surgery could enhance the outcome. Platelets, when used as adjunctive treatment, have shown good results. However, laser biostimulation post-surgery has not been studied.
Objectives. The present study compared the benefits of using the conventional and laser-assisted flap techniques with platelet-rich fibrin (PRF) in the treatment of class I and class II gingival recession.
Material and methods. The study included 24 subjects, both males and females. The participants, diagnosed with Miller’s class I and II gingival recession, were categorized into 2 groups: group A (n = 12) treated with CAF and PRF; and group B (n = 12) treated with laser-assisted CAF and PRF. Root coverage (RC), the probing depth (PD), the clinical attachment loss (CAL), and the keratinized tissue width (KTW) were assessed pre- and up to 6 months postoperatively. The wound healing index (WHI) and the visual analog scale (VAS) score were evaluated 1 week post-surgery.
Results. Most clinical parameters improved significantly within the groups at 6 months postoperatively as compared to baseline (p < 0.05), except for PD and percentage root coverage (PRC). However, when intergroup comparisons were made, it was observed that both groups performed equally well and the differences between them were not significant.
Conclusions. Both treatment modalities improved the clinical parameters post-surgery. However, further trials are warranted to affirm the benefits of the laser-assisted CAF technique.
Keywords: platelet-rich fibrin, gingival recession, coronally advanced flap, laser biostimulation
Introduction
Gingival recession is a highly prevalent problem among adults. It can cause esthetic impairment, dentin hypersensitivity, root abrasion, and root caries.1, 2, 3 The key reason for this condition is inflammation within the marginal gingival connective tissue; others include traumatic toothbrushing, the frenal pull and the malposition of the teeth.4 It is always important to correct the malposition of the teeth orthodontically before attempting recession coverage; otherwise, the treatment will not be successful. Many classifications are available to grade gingival recession, and it has been observed that the prognosis for grade 1 and grade 2 gingival recession is good. Free autogenous grafts and pedicle grafts, such as coronally advanced flaps (CAFs) and semilunar flaps, have been employed to treat recession. Among these techniques, CAF is the commonly used procedure to reposition the gingiva in the coronal direction, providing good clinical outcomes.5, 6, 7 Acellular dermal matrices have also been used for root coverage (RC) with good results.8 Various platelet concentrates have been applied in dentistry, including pure platelet-rich plasma (P-PRP), leukocyte- and platelet-rich plasma (L-PRP) and platelet-rich fibrin (PRF), which in turn comprises pure platelet-rich fibrin (P-PRF), leukocyte- and platelet-rich fibrin (L-PRF) and injectable platelet-rich fibrin (I-PRF).9
Platelet-rich fibrin plays a vital role in the regeneration of the lost bone and soft tissues. This biomimetic agent has better healing properties than other platelet concentrates, which justifies its use in mucogingival procedures and the implant therapy.10, 11 A laser-assisted releasing incision in the vestibule after surgery aids in decreasing tension on the flap and promotes better healing of the surgical site. Mucogingival procedures are very technique-sensitive and patients often experience pain after surgery. The low-level laser therapy (LLLT) alleviates gingival inflammation, decreases pain and promotes wound healing.12 Hence, the present study compared the efficacy of conventional and laser-assisted CAFs with PRF in RC.
Material and methods
Preliminary plan and ethics statement
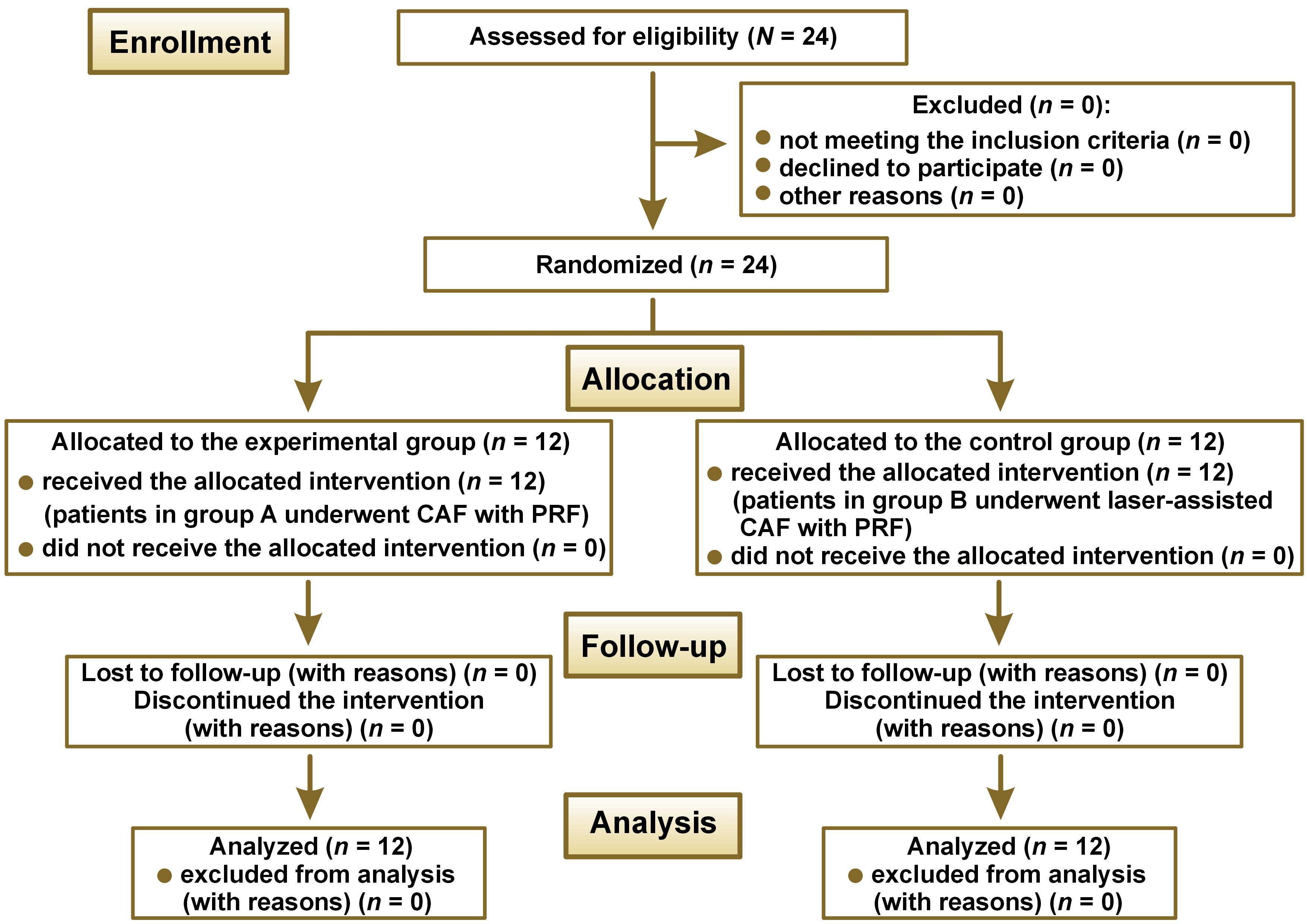
The study was designed as a randomized, parallel-arm clinical trial comparing the efficacy of RC with the use of the conventional vs. laser-assisted flap technique with PRF in the treatment of class I and class II gingival recession. It included 24 patients and was conducted at the outpatient ward of a tertiary referral care center in Hyderabad, India. The study was carried out from May 2019 to October 2020. It complied with the ethical standards established by the World Medical Association (WMA) in the Declaration of Helsinki, and was approved by the institutional ethics committee at the Panineeya Institute of Dental Sciences & Research Centre, Hyderabad, India (PMVIDS&RC/IEC/PERIO/DN/0218-2018). All participants were given a detailed verbal and written description of the study, and a signed consent form was obtained from each of them. The flowchart of the study design is presented in Figure 1.
Inclusion and exclusion criteria
Patients with Miller’s class I and II recession defects on the anterior teeth, with the probing depth (PD) >3 mm, the clinical attachment loss (CAL) >5 mm and the keratinized tissue width (KTW) >2 mm, were included in the study. Pregnant and lactating women, smokers, systemically compromised patients, subjects who had undergone the periodontal therapy in the last 6 months, and those who were on antibiotics 3 months prior to commencing the study (confirmed while recording the case history) were excluded from the study.
Sample size calculation
As per a statistician’s suggestion, to obtain a difference in complete root coverage (CRC) between the groups with a power of 80% and a 95% confidence interval (CI), 12 patients had to be included in each group. The primary outcome variables assessed were the recession depth (RD), the gingival thickness (GT) and percentage root coverage (PRC), whereas PD, CAL, KTW, the visual analog scale (VAS) score, and the wound healing index (WHI) were the secondary outcomes measured.
Estimation of the clinical parameters
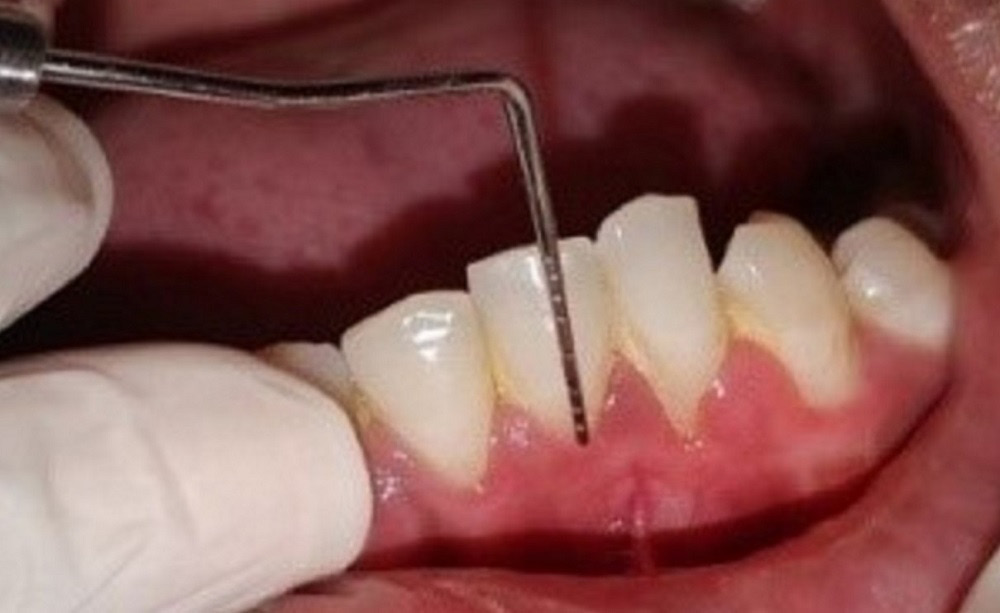
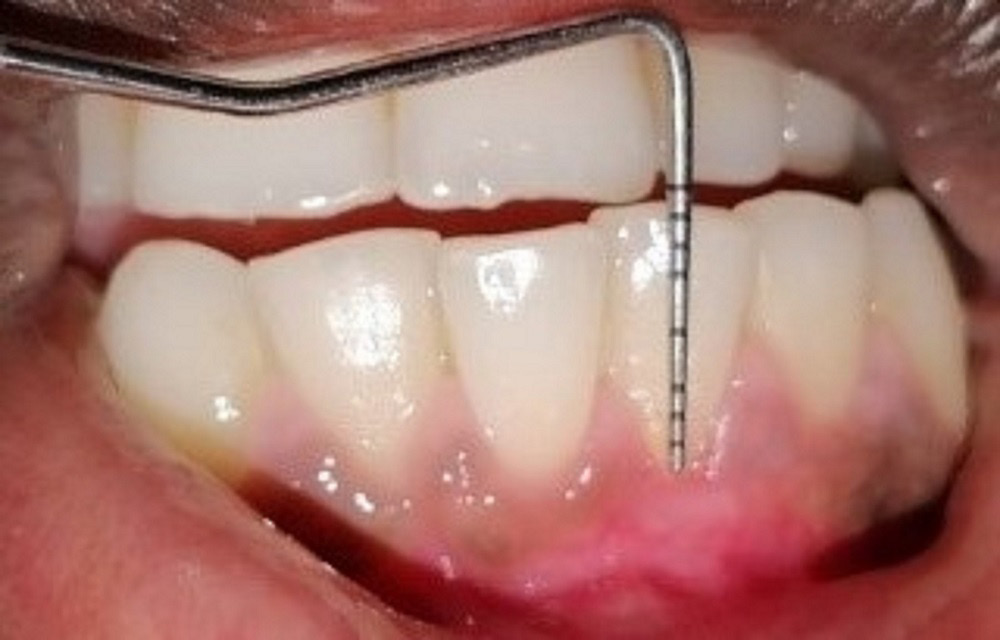
The recession depth, GT, PD, CAL, and KTW were assessed using a Williams probe at baseline (D0), and 3 months (D3) and 6 months (D6) post-surgery, whereas PRC was assessed at 3 months (D3) and 6 months (D6) post-surgery. The VAS score and WHI were estimated 1 week after surgery.
Randomization
One investigator (R.R.K.) assigned the cases by randomly picking them out from the sealed envelopes, and the other investigator (M.B.) performed the surgeries for both groups. Both the patients and the statistician were blinded to the assignment.
Groups
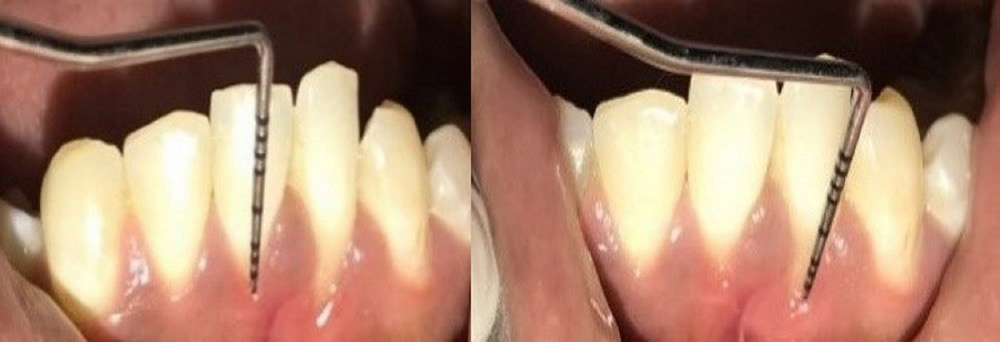
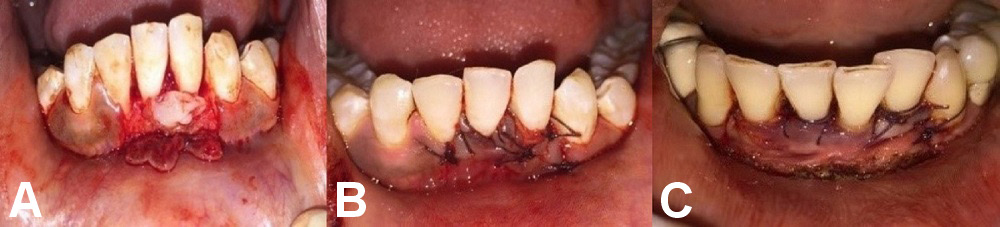
Group A comprised 12 patients who underwent surgery to treat their denuded roots by CAF and PRF application (Figure 2).
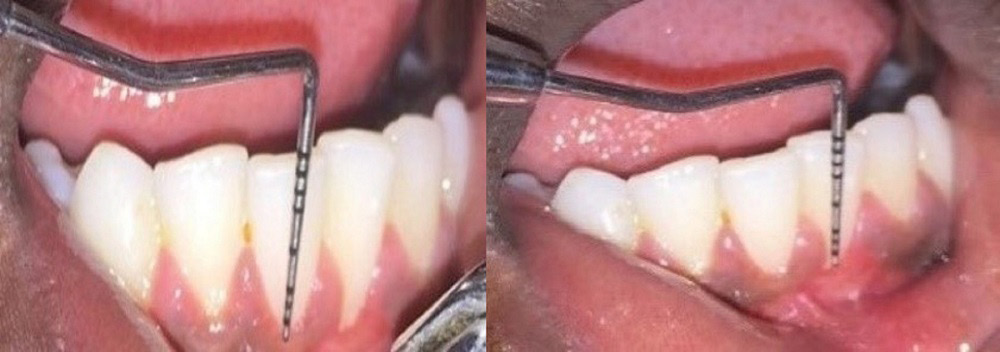
Group B comprised 12 patients who underwent laser-assisted RC by CAF and PRF application (Figure 3).
Presurgical procedure
The patients initially received a comprehensive periodontal examination and a complete plaque control program, including oral hygiene, to eliminate the habits related to the etiology of recession. Scaling, root planing and occlusal adjustments were performed 1 month before the surgical protocol was implemented.
Surgical procedure
The patient was seated comfortably on a dental chair, and then asked to rinse their mouth with a 1:1 ratio of 0.2% chlorhexidine digluconate solution. The operation site was anesthetized with 2% lignocaine hydrochloride with adrenaline (1:80,000), using the block and infiltration techniques.
Preparation of the platelet-rich fibrin membrane
Platelet-rich fibrin was harvested from a simple blood sample (2 mL) drawn from the patient’s antecubital vein at the time of the surgical procedure. It was then treated with single centrifugation at 2,700 rpm for 12 min. After the centrifugation procedure, 3 distinct layers were formed, of which the intermediate layer was that of a dense PRF clot. The fibrin clot was easily separated from the red blood cell (RBC) base by using sterile tweezers and scissors, with the preservation of the 2 other RBC layers. This dense PRF clot was used as a membrane.
Group A
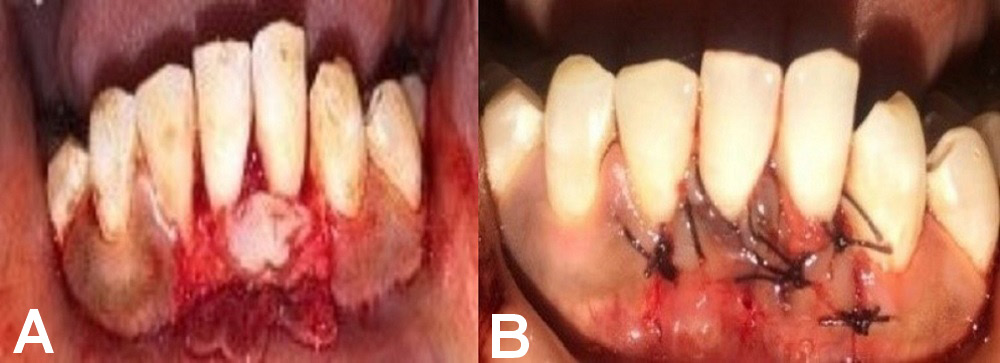
A full-thickness submarginal trapezoidal flap was raised with the use of blade No. 15 on the labial aspect of the tooth being treated, through an intrasulcular incision extending horizontally to dissect the labial aspect of the adjacent papilla. Two vertical incisions were made – one at the distal gingival line angle, and the other at the mesial line angle of the subject’s affected tooth. The submarginal horizontal incisions connected with the vertical incisions were extended up to the mucogingival junction (MGJ) to provide the proper displacement of the flap. The flap was raised through sharp dissection. The papillae were de-epithelialized. Before placing the pedicle flap on the denuded root, thorough root planing was performed using curettes, and the prepared PRF membrane was placed and sutured using 4–0 resorbable sutures. The pedicle flap was then sutured 1 mm coronal to the cementoenamel junction (CEJ) of the affected tooth, using 4–0 resorbable sutures. A COE-PACK™ dressing was applied (Figure 4).
Group B
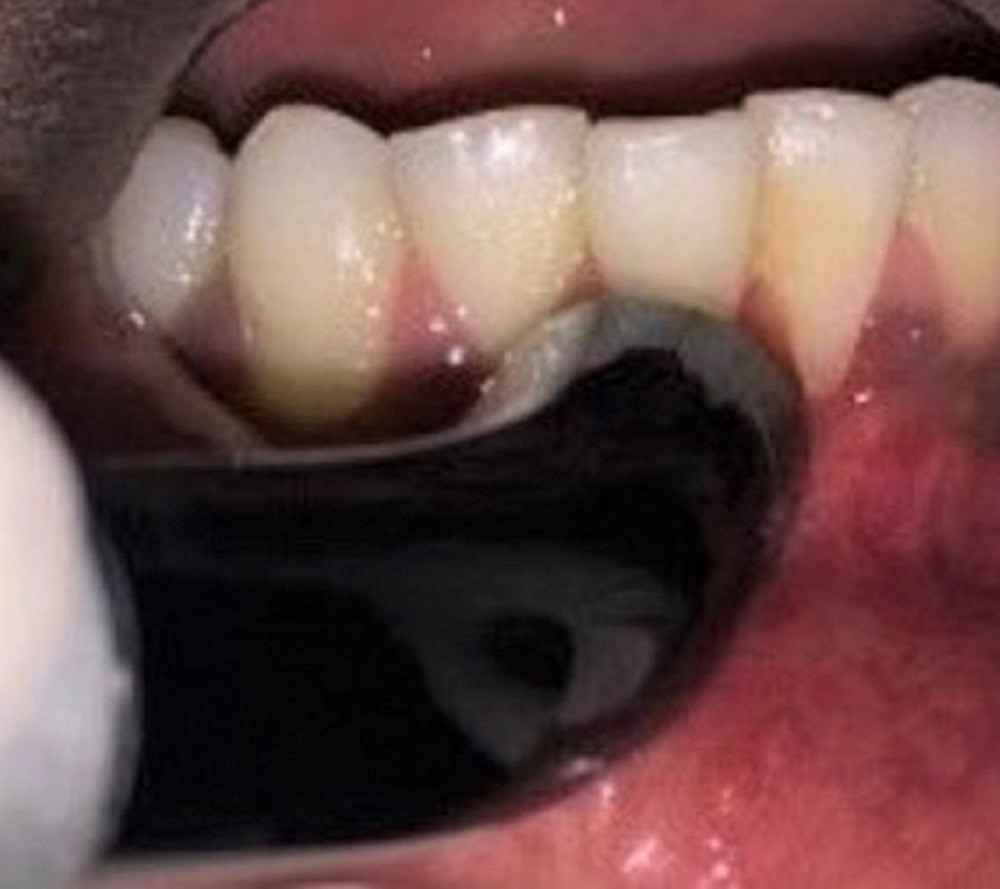
A similar surgical protocol was followed in group B, but an additional vestibular releasing incision was made using an optical-fiber diode laser at a power of 3 W in the continuous mode (Figure 5). This procedure helped prevent muscle reattachments that could hinder the outcome of the treatment. Photobiostimulation was performed at the end of the RC procedure and on day 7 with a 980 nm diode laser at a power of 1 W in the continuous mode for 60 s (Figure 6).
Postoperative protocol
The patients were advised to take an analgesic (aceclofenac 100 mg, 3 times a day for 5 days) and an antibiotic (amoxicillin 500 mg, 3 times a day for 5 days) post-surgery, and instructed to refrain from tooth brushing and flossing until the removal of the sutures. They were also instructed to rinse their mouth with chlorhexidine mouthwash (0.12%) twice daily for a period of 1 month. The sutures were removed 7 days post-surgery (Figure 7 and Figure 8).
Each patient was reinstructed about proper oral hygiene measures, and was recalled after 1 week and thereafter monthly until the end of the 6th month. Scaling and oral hygiene reinforcement were provided at each follow-up visit whenever indicated until the 6th month.
Statistical analysis
The data was analyzed using the Microsoft Excel and GraphPad Prism software. The continuous data was summarized as mean and standard deviation (M ±SD). The intragroup comparisons were performed using the repeated one-way analysis of variance (ANOVA) for continuous data, followed by Bonferroni’s multiple comparison test. The intergroup comparisons were performed using the repeated two-way ANOVA for continuous data. All p-values less than 0.05 were considered statistically significant.
Results
Group A
The mean RD at D0, D3 and D6 was 2.54 mm, 1.96 mm and 1.70 mm, respectively. It was observed that the RD values decreased significantly at D6 as compared to D0 (p < 0.001). The mean CAL at D0, D3 and D6 was 4.19 mm, 3.49 mm and 3.19 mm, respectively, and the values also decreased significantly between D0 and D6 (p < 0.001). The mean GT at D0, D3 and D6 was 1.43 mm, 1.53 mm and 1.57 mm, respectively, and the GT values increased significantly from D0 to D3 and from D3 to D6 (p < 0.05). The mean KTW at D0, D3 and D6 was 1.97 mm, 2.65 mm and 2.93 mm, respectively. There was a significant increase in the KTW values from D0 to D6 (p < 0.05). The mean PD at D0, D3 and D6 was 1.65 mm, 1.43 mm and 1.47 mm, respectively. There was no significant change from D0 to D6 for the PD values (p = 0.127). The mean PRC at D3 and D6 was 22.63% and 34.00%, respectively. There was no significant change in the PRC values between D3 and D6 (p = 0.131). Therefore, there was improvement at different time points for the parameters RD, GT, CAL, and KTW, but the PD and PRC values did not improve (Table 1).
Group B
The mean RD at D0, D3 and D6 was 2.30 mm, 1.91 mm and 1.63, respectively. There was a significant decrease at D6 as compared to D0 (p < 0.05). The mean GT at D0, D3 and D6 was 1.42 mm, 1.50 mm and 1.65 mm, respectively. There was significant improvement in GT from D0 to D6 (p < 0.05). The mean PD at D0, D3 and D6 was 1.96 mm, 1.60 mm and 1.69, respectively. There was a significant change in the PD values from D0 to D6 (p < 0.05). The mean KTW at D0, D3 and D6 was 2.06 mm, 2.66 mm and 2.85 mm, respectively. There was a significant increase in the KTW values from D0 to D6 (p < 0.05). The mean CAL at D0, D3 and D6 was 4.30 mm, 3.45 mm and 3.26 mm, respectively. There was a significant decrease in CAL from D0 to D6 (p < 0.05). The mean PRC at D3 and D6 was 30.69% and 39.37%, respectively. There was no significant change in the PRC values between D3 and D6 (p = 0.145). Therefore, there was improvement at different time points for the parameters RD, GT, PD, CAL, and KTW, but the PRC values did not improve (Table 2).
Intergroup comparison of RD, GT and PD in groups A and B
The mean RD in group A at D0, D3 and D6 was 2.54 mm, 1.96 mm and 1.70 mm, respectively, and in group B, it was 2.30 mm, 1.91 mm and 1.63 mm, respectively. Upon intergroup comparison, the differences in results were not significant. The mean GT in group A at D0, D3 and D6 was 1.43 mm, 1.53 mm and 1.57 mm, respectively, and in group B, it was 1.42 mm, 1.50 mm and 1.65 mm, respectively, showing no significant differences upon intergroup comparison. The mean PD in group A at D0, D3 and D6 was 1.65 mm, 1.43 mm and 1.47, respectively, and in group B, it was 1.96 mm, 1.60 mm and 1.69 mm, respectively, showing no significant differences between the groups (Table 3).
Intergroup comparison of CAL, KTW and PRC in groups A and B
The mean CAL in group A at D0, D3 and D6 was 4.19 mm, 3.49 mm and 3.19 mm, respectively, and in group B, it was 4.30 mm, 3.45 mm and 3.26, respectively, with no significant differences between the groups. The mean KTW in group A at D0, D3 and D6 was 1.97 mm, 2.65 mm and 2.93 mm, respectively, and in group B, it was 2.06 mm, 2.66 mm and 2.85 mm, respectively, showing no significant differences. The mean PRC in group A and group B at D3 was 22.63% and 30.69%, respectively, and at D6, it was 34.00% and 39.37%, respectively. Thus, the CAL, KTW and PRC values also did not show statistically significant differences upon intergroup comparison (Table 4).
Intergroup comparison of the VAS scores and WHI in groups A and B
The mean VAS score at 1 week for group A was 2.29, and for group B, it was 2.17. The mean WHI value at 1 week for group A was 3.13, and for group B, it was 3.42. There were no significant differences between the groups for either of these parameters (Table 5).
Discussion
Gingival recession is a very common problem associated with esthetic and functional impairment. Thus, the achievement of CRC is the goal of every clinician.13
The CAF technique as a treatment option was introduced by researchers nearly a century ago. Thereafter, other modalities of RC were implemented. The proper case selection is vital for obtaining a successful outcome. The parameters to be assessed include RD, the recession width (RW), the vestibular depth, KTW, the width and height of the interdental soft tissue, and the insertion of the frenulum.14, 15
The present study was conducted to evaluate the efficacy of a conventional CAF with PRF vs. a laser-assisted CAF with PRF in the treatment of class I and class II gingival recession. Laser biomodulation was implemented after the laser-assisted CAF; this study is among the few studies that have examined RC, employing this principle.
The CAF technique as a treatment option has yielded very good results pertaining to PRC, as well as color matching to the adjacent tissues.16 Moreover, it has been proven to be efficacious in the treatment of multiple gingival recessions, with good esthetic results.17
Platelet-rich fibrin helps increase the efficacy of CAF, as PRF is rich in growth factors that are released within 7–28 days. The membrane acts as a barrier that prevents the ingress of gingival epithelial cells into the defect. Furthermore, it plays a direct role in increasing angiogenesis and modulating tissue healing, and helps ward off inflammation.18, 19
Pioneers in research related to PRF conducted a biological assay.20 They performed a comparative study to evaluate the roles of transforming growth factor beta 1 (TGFβ-1), insulin-like growth factor 1 (IGF-1), platelet-derived growth factor (PDGF-BB) in the platelet-poor plasma (PPP) supernatant, and the PRF clot exudate serum. The study revealed that PRF slowly polymerized fibrin, leading to the incorporation of glycanic chains and platelet concentrates in the fibrin mesh. Hence, PRF has the added advantage of a continuous, slow release of cytokines during tissue remodeling, thus accelerating wound healing.20
Eren and Atilla conducted a split-mouth trial wherein 22 patients with class I or class II gingival recession participated.21 Forty-four defects were evaluated after receiving either CAF with PRF (test group: 22 defects) or CAF with the subepithelial connective tissue graft (SCTG) (control group: 22 defects). All the clinical parameters pertaining to RC were assessed preoperatively and 6 months postoperatively. The recession depth, RW, the recession area (RA), and KTW were calculated on standardized photographs, using digital image analysis software. The results indicated that both groups performed equally well, with no significant differences between them, prompting the use of PRF as an alternative to SCTG in localized gingival recessions.21
Another clinical study was conducted to ascertain the potential benefits of using PRF with the modified CAF for the treatment of gingival recession.22 For this split-mouth research, 12 patients with Miller’s class I and class II gingival recession in 2 non-adjacent anterior teeth were chosen. One tooth with gingival recession was subjected to the modified CAF, while the other was treated with CAF with PRF. The changes in parameters (RD, RW, GT, and CAL) from 1 month to 3 months and 6 months within the groups were statistically non-significant. Upon intergroup comparison, only the change in GT was found to be statistically significant with regard to the CAF + PRF group.22
Potey et al. conducted a split-mouth trial to evaluate and compare the effectiveness of CAF with or without the use of the PRF membrane in the treatment of multiple adjacent recession defects (MARD), clinically and by means of cone-beam computed tomography (CBCT).23 Twenty healthy patients having 75 MARD were allocated randomly to the CAF + orthodontic button group (CAFB) or the CAFB + PRF membrane group (CAFB + PRF). No notable differences were observed between the groups regarding PRC, RD, PD, or CAL. The use of PRF resulted in a highly significantly increased GT at 6 months, indicating that CAFB can be successfully used to treat MARD with predictable outcomes.23
Bhattacharya et al. performed a study to assess the efficacy of PRF collected in silica (SiO2) tubes or titanium (Ti)-coated tubes, using immunohistochemistry (IHC).24 The results showed that in the Ti group, the IHC staining was better, with more T cells, B lymphocytes and platelets. It was concluded that Ti PRF was better than silica PRF in periodontal regeneration.24
In the current study, the use of PRF resulted in improved RD, GT, PD, CAL, and KTW in both groups.
The laser-assisted CAF procedure was used in the patients allocated to group B. A laser-assisted vestibular releasing incision was made (a 980 nm diode laser at a power of 3 W). A relieving incision was made superficially, 7 mm apical to the gingival margin, which minimized tension on the flap, and also prevented muscle reattachments during the tissue remodeling phase. Due to the dual advantage of this technique, it is anticipated that RC would be better and more stable.25 However, no documented studies are available to report the benefits of the laser vestibular releasing incision approach.
It was observed in the current study that the patients in group B performed equally well as those allocated to group A.
The benefits of using LLLT to facilitate wound healing have been reported in many studies. The therapy consists in biostimulation or biomodulation, and is based on the principle that irradiation at a specific wavelength is able to alter cellular behavior. The mitochondrial cellular respiratory chain is stimulated and the membrane calcium (Ca) channels are activated, thus enhancing tissue metabolism and proliferation.26
The major changes observed in the wounds treated with LLLT include increased granulation synthesis, enhanced neovascularization of tissue, and increased fibroblast proliferation, maturation, attachment, and matrix synthesis. The tensile strength of flap margins after LLLT has been reported to increase, preventing the collapse of the healing wound, and thus minimizing soft tissue recession.27
In another study comparing outcomes when using an additional external vestibular releasing incision made with a diode laser or a scalpel along with a laterally positioned flap, the authors reported reduced patient discomfort in the laser incision group.28
A systematic review conducted by Yan et al. showed that after applying light irradiation to gingival recession, the outcomes in terms of CRC ranged from 70% to 90%.29 Thus, a higher CRC was found in the experimental group as compared to the group that did not undergo laser treatment following surgery. However, the pooled analysis from this meta-analysis related to CRC found that there was no significant difference between the 2 groups based on the 6- and 12-month results (p = 0.300 and p = 0.160, respectively).29 In accordance with the results of the review, CRC in the present study was similar between the 2 groups at 6 months postoperatively. A trend regarding the shift of gingival margins over time has been observed in previous studies.30, 31
Aleksić et al. conducted a study to evaluate WHI with regard to the use of PRF and the connective tissue graft (CTG) in the treatment of recession defects.32 Less patient discomfort and better acceptance with faster healing were observed in the PRF group as compared to the CTG group.32 In the present study, the mean WHI in the conventional and laser groups at 1 week was 3.13 and 3.42, respectively, showing no significant difference. The results could be due to the use of the PRF membrane, which is known to release various pro-inflammatory cytokines, such as interferon gamma (IFγ), tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL-1β), and IL-6, which help in T cell differentiation, and growth factors, such as vascular endothelial growth factor (VEGF), PDGF, fibroblast growth factor (FGF), TGF, and IGF, which act as anti-inflammatory agents and promote faster healing.33
Pain control following the operation is a necessary part of periodontal treatment. This pain results from tissue trauma and the release of inflammatory mediators, and reaches its highest peak following the cessation of local anesthesia. The analgesic mechanism of low-level laser is not yet clear. However, several studies have pointed out that light interference may be the cause of physiological changes in numerous cell types. Low-level laser can modify the inflammatory process in a dose-related manner; hence, it can reduce inflammatory pain. In acute pain, the optimal outcome is reached when low-level laser is prescribed within the first 72 h following the operation.34
Another split-mouth randomized clinical trial (RCT) evaluated the benefits of laser application in 12 patients after palatal graft harvesting.35 In the test group, following the free gingival graft procedure, a 660 nm diode laser with a power of 200 mW was applied to the target site for 32 s. This was repeated on days 1, 2, 4, and 7 postoperatively. In the control group, sham laser was used in the same way. To evaluate the amount of epithelialization and for clinical repair observations, photographic images were used. The amount of sedative drugs taken was recorded to assess the pain scale on day 14. The palatal wound in the laser-applied group was significantly better healed than in the control group regarding clinical repair and epithelialization. Then, on day 21, the amount of epithelialization was significantly better in the laser-applied group than in the control group. However, the 2 groups showed no significant differences in the amount of sedative drug used and bleeding. The authors concluded that low-level laser might heal the wound in the palatal graft site.35
In the current study, the VAS scores were assessed in both group A and group B 1 week postoperatively. It was observed that in group A, the score was 2.29, and in group B, it was 2.17, showing no significant difference between the groups.
Hence, the present study showed improvement in RD, GT, CAL, and KTW, but the differences in the values of these parameters between the groups were not statistically significant. The obtained results are in accordance with earlier studies.36, 37 However, the PRC values did not show significant improvement in this study, which was also observed in previous research.38
Limitations
The follow-up period for the groups in this study was 6 months. The results could have been more significant if the follow-up had been carried out for 9 months.
Conclusions
This study showed improvement in RD, GT, CAL, and KTW, although both groups performed equally well. Perhaps the validity of the study could have been strengthened by increasing the sample size as well as conducting the study for a longer period of time. The benefits of using laser for both the vestibular releasing incision and biomodulation have to be confirmed by conducting more clinical trials.
Ethics approval and consent to participate
The research was approved by the institutional ethics committee at the Panineeya Institute of Dental Sciences & Research Centre, Hyderabad, India (PMVIDS&RC/IEC/PERIO/DN/0218-2018). Each participant provided written informed consent to participate in the study.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.