Abstract
Background. Many patients are clinically consulted in dental clinics, where aerosol-generating procedures are widely used. In our previous study, we evaluated the temperature, humidity and contamination rates on the inner layer of masks according to the mask-wearing time. However, it is important to assess the contamination rates on the outer layer of masks used in dentistry as well. Previously, while examining the contamination rates, we only identified the associated bacteria; no detailed analysis of bacterial species depending on the mask-wearing time was conducted. Furthermore, we did not evaluate factors that could contribute to the contamination of masks.
Objectives. The present study was intended to supplement the limitations of our previous study.
Material and methods. The used masks were collected. Thereafter, colony forming unit (CFU) quantification and 16S rDNA sequencing were performed to calculate the contamination rates and identify bacterial species. Data on the participants’ medical and dental history was collected. The participants filled out a questionnaire and underwent saliva tests.
Results. On the inner and outer layers of the masks, 3.3 × 108 and 8.5 × 108 CFUs were found, respectively. The contamination rates of the masks increased with the increasing mask-wearing time. There was no correlation between the contamination rate on the inner layer and other factors, such as the probing depth (PD) ≥4 mm, the bleeding rate, the calculus rate, and saliva characteristics. The inner layer contamination rate increased as the number of treated teeth increased, and as the saliva buffering capacity decreased. The outer layer contamination rate increased with the number of times the mask was touched.
Conclusions. The contamination rates were higher on the outer layer than on the inner one, and the CFU count increased with the mask-wearing time. The following bacterial species were found on the masks: Staphylococcus epidermidis (S. epidermidis); Staphylococcus aureus (S. aureus); Staphylococcus capitis (S. capitis); Streptococcus oralis (S. oralis); and Streptococcus koreensis (S. koreensis). Oral health conditions may have affected the contamination of the inner layer. In addition, the number of times the mask was touched may have affected the contamination of the outer layer.
Keywords: bacterial contamination, mask contamination, mask contamination factors
Introduction
Wearing a mask is crucial for protecting the individual’s health in a dental clinic. It has become an essential safety measure during the coronavirus disease 2019 (COVID-19) pandemic.1 Particularly, in dental clinics, clinicians have to consult many patients and aerosol-generating procedures are widely used. Consequently, the mask contamination rates may increase in such settings.2 However, hospital guidelines for infection prevention lack evidence for mask replacement cycles.3 Hence, in our previous study, we evaluated the temperature, humidity and contamination rates on the inner layer of masks according to the mask-wearing time.4 Yet, it is important to assess the contamination rates on the outer layer of masks used in dentistry as well. No studies have referred to this issue so far. In addition, in our previous study, we only identified the associated bacteria; no detailed analysis of bacterial species depending on the mask-wearing time was conducted. Furthermore, we did not evaluate factors that could contribute to the contamination of masks.
Therefore, the present study was intended to supplement the limitations of our previous study.
Material and methods
Participants
This study was approved by the Ethics Committee of Ulsan College, South Korea (No. 1044363-A-2022-002). The power analysis was performed for the study (effect size: d = 0.5, an alpha (α) error of 0.05 and a power of 0.95) with the use of G*Power, v. 3.1.9.7 (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower). The total sample size required to obtain conclusive results was 54, and a sample size of 100 was selected to compensate for possible error-related dropouts. Then, 100 participants who provided written informed consent after the study purpose had been explained to them were recruited. Finally, 91 participants (mean age: 28.3 ±0.3 years, no medical history, free of medications) were included for further analysis.
Sample collection
Clinicians, including dentists, dental hygienists and nursing assistants, were selected as participants in this study. The participants responded to a questionnaire including questions about the mask-wearing time, whether they chewed gum, coughed or sneezed while wearing the mask, and whether they washed their faces, brushed their teeth or put on makeup before using the mask. We also recorded behavioral factors, such as the number of times the mask was touched. After completing the survey, the masks were collected, placed in sterile zipper bags and sent to the laboratory. Sample numbers were indicated on the questionnaires and outside the zipper bags to ensure the anonymity of the participants.
Quantification of colony forming units (CFUs)
After collecting the masks, a square shape (1 cm × 1 cm) in the center of each mask was cut out, and the inner (inside) and outer layers (outside) were separated using tweezers. The separated pieces were placed in Eppendorf tubes containing 1 mL of the Luria–Bertani medium, vortexed for 1 min and incubated at 37°C for 24 h. After dilution (from 10−1 to 10−6), aliquots of 100 µL were inoculated onto plate count agar (PCA) plates. After overnight incubation at 37°C, CFUs were counted.
Identification of bacteria
The colonies on the PCA plates were picked randomly and subcultured 3 times. Polymerase chain reaction (PCR) was implemented using a paired primer set (forward primer: 27F: AGA GTT TGA TCM TGG CTC AG; reverse primer: 1492R: GGT TAC CTT GTT ACG ACT TC). The reaction mixtures were subjected to 30 cycles of denaturation and annealing at 50°C in an automated thermal cycler (SimpliAmp™; Thermo Fisher Scientific, Waltham, USA). The PCR products were resolved by electrophoresis on 1% agarose gel containing ethidium bromide (EtBr). The final PCR products were purified using a DNA purification kit (LaboPass™; Cosmo Genetech Co., Ltd., Seoul, South Korea). Finally, the PCR products were analyzed with automated sequencing, using a cycle sequencing kit (BigDye™ Terminator, v. 3.1; Thermo Fisher Scientific), a sequencing machine (ABI 3730XL; Thermo Fisher Scientific) and the Sequencher DNA sequence analysis software, v. 5.2 (Gene Codes Corp., Ann Arbor, USA). The newly aligned 16S rDNA sequences were compared with the bacterial genes deposited in the GenBank® database (National Center for Biotechnology Information (NCBI), Bethesda, USA), and the bacterial strains with a match of 99% were searched using the Basic Local Alignment Search Tool (BLAST) for nucleotides (NCBI).5 If alignment with the 27F and 1492R primers failed, additional experiments were conducted using an internal primer (800R: TAC CAG GGT ATC TAA TCC; 785F: GGA TTA GAT ACC CTG GTA), and if this alignment failed as well, 5.8S rRNA sequencing (ITS1: TCC GTA GGT GAA CCT GCG G; ITS4: TCC TCC GCT TAT TGA TAT GC) and 25–28S rRNA sequencing (NL1: GCA TAT CAA TAA GCG GAG GAA AAG; NL4: GGT CCG TGT TTC AAG ACG G) were performed additionally.
Medical and dental history taking
All participants underwent intraoral examinations, and one investigator collected data on the participants’ medical and dental history, medication, and progressive oral diseases, such as dental caries. The investigator also measured attrition and abrasion, the probing depth (PD), the bleeding rate and the calculus rate.
Saliva test
Five minutes after the participants brushed their teeth, their saliva was collected into 15-milliliter conical tubes for 5 min while sitting upright and comfortably on a chair. After that, the amount and pH of saliva were measured using a pH meter (PH-200, HM Digital, Inc., Carson, USA). After chewing gum for 30 s, the saliva secreted for 5 min was collected into a measuring cup to determine its amount. The secretion rates of unstimulated and stimulated saliva were recorded. The saliva buffering capacity was measured using a saliva-check buffer kit (GC Corp., Tokyo, Japan). After dropping the collected saliva onto the tester, the color change was read. The determination of the color change was conducted by 3 independent investigators, and the average was calculated.
Statistical analysis
The SPSS for Windows software, v.12.0 (SPSS Inc., Chicago, USA), was used for statistical analysis. The data was evaluated with the one-way analysis of variance (ANOVA) followed by the paired t test. The results of the experiments are represented as mean and standard error (M ±SE). A p-value <0.05 was considered statistically significant.
Results
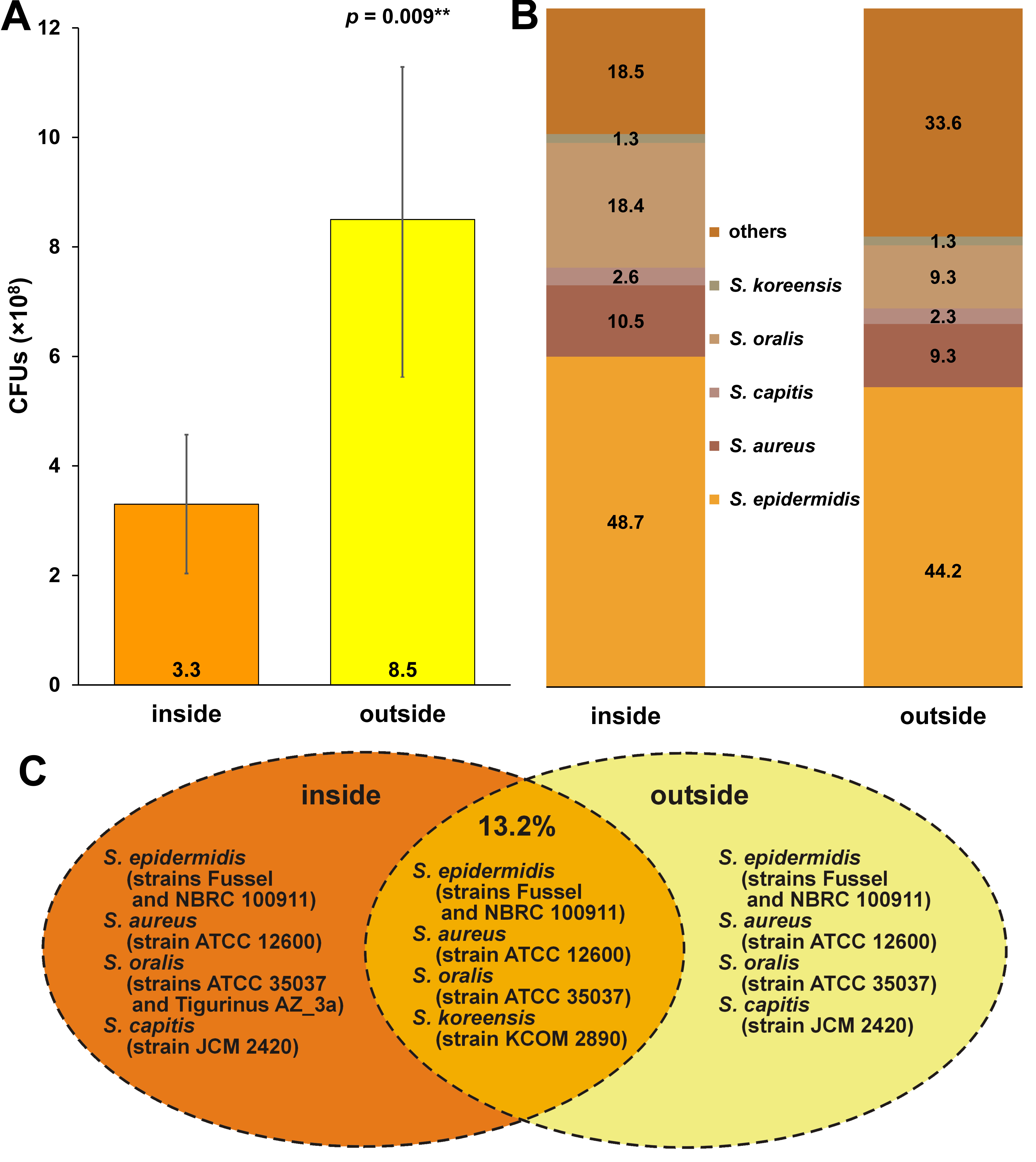
The average CFU count on the inner and outer layers of the masks was 3.3 × 108 and 8.5 × 108, respectively, and the difference was statistically significant (p = 0.009) (Figure 1). On both sides of the masks, the following bacterial species were found: Staphylococcus epidermidis (S. epidermidis) (inside: 48.7%; outside: 44.2%); Staphylococcus aureus (S. aureus) (inside: 10.5%; outside: 9.3%); Staphylococcus capitis (S. capitis) (inside: 2.6%; outside: 2.3%); Streptococcus oralis (S. oralis) (inside: 18.4%; outside: 9.3%); and Streptococcus koreensis (S. koreensis) (inside: 1.3%; outside: 1.3%) (Figure 1). Furthermore, Streptococcus salivarius (S. salivarius) (7.9%), Staphylococcus lugdunensis (S. lugdunensis) (2.8%), Streptococcus infantis (S. infantis) (1.3%), Streptococcus rubneri (S. rubneri) (1.3%), Streptococcus australis (S. australis) (1.3%), Streptococcus sanguinis (S. sanguinis) (1.3%), Streptococcus parasanguinis (S. parasanguinis) (1.3%), and Moraxella osloensis (M. osloensis) (1.3%) were found on the inner layer of the masks only (Figure 1, ‘others’ in the inside group). In contrast, Staphylococcus warneri (S. warneri) (18.6%), Staphylococcus caprae (S. caprae) (2.5%), Streptococcus mitis (S. mitis) (2.5%), Enterococcus faecium (E. faecium) (2.5%), Psychrobacillus lasiicapitis (P. lasiicapitis) (2.5%), Bacillus thioparans (B. thioparans) (2.5%), and Lactobacillus plantarum (L. plantarum) (2.5%) were found on the outer layer of the masks only (Figure 1, ‘others’ in the outside group). On the inner and outer layers, 13.2% of the bacteria found were of the same strains (S. epidermidis: strains Fussel and NBRC (National Institute of Technology and Evaluation (NITE) Biological Resource Center) 100911; S. aureus: strain ATCC (American Type Culture Collection) 12600; S. oralis: strain ATCC 35037; and S. koreensis: strain KCOM (Korean Collection for Oral Microbiology) 28901), while for the rest of the bacteria (S. epidermis, S. aureus, S. oralis, and S. capitis), the strains were different, even though the species were the same (Figure 1).
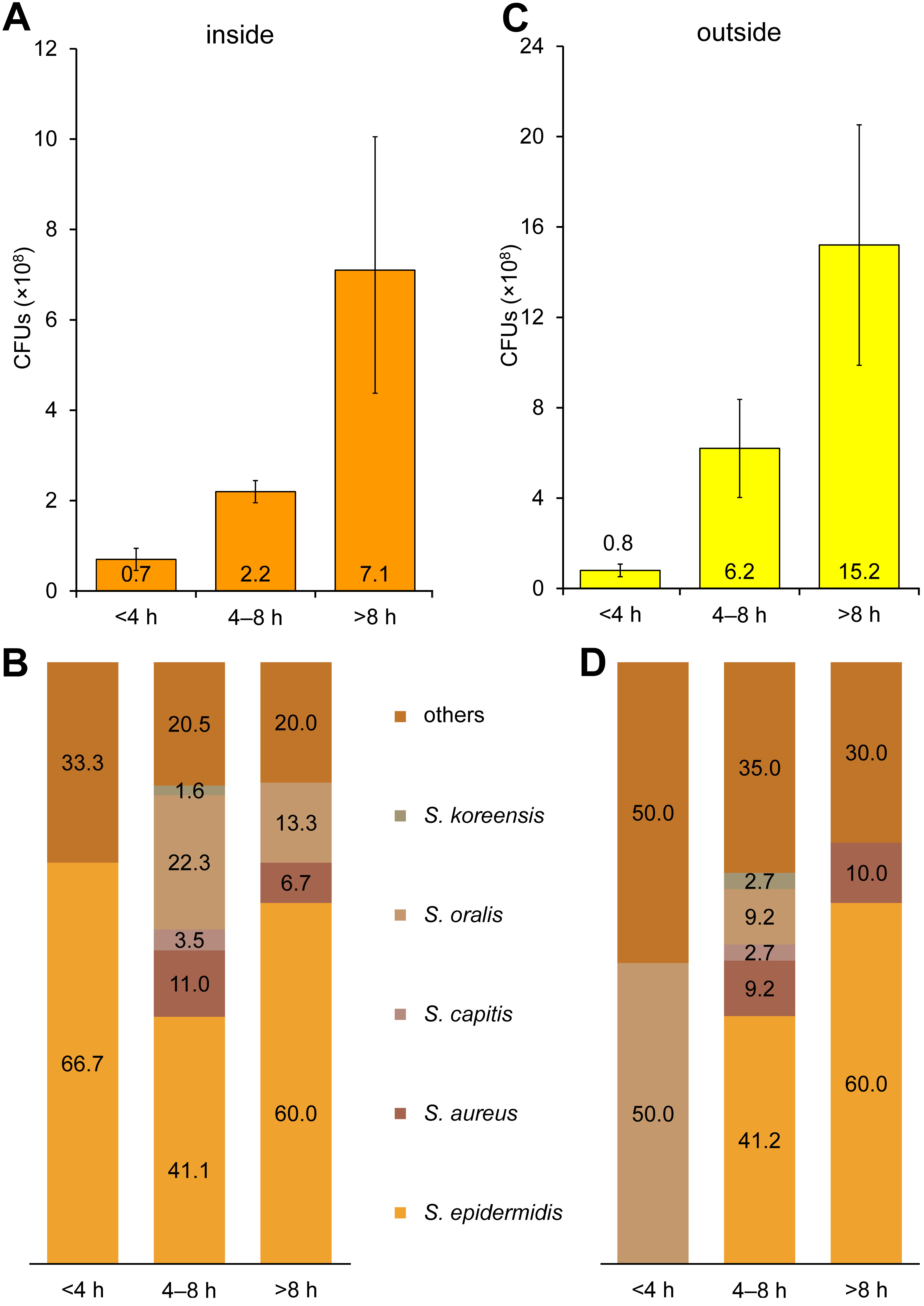
The contamination rates on the inner layer increased as the mask-wearing time increased (<4 h: 0.7 × 108 CFUs; 4–8 h: 2.2 × 108 CFUs; and >8 h: 7.1 × 108 CFUs) (Figure 2). When the mask-wearing time was less than 4 h, most bacteria found were S. epidermidis (66.7%). Staphylococcus epidermidis (41.1%), Streptococcus aureus (11.0%), S. capitis (3.5%), S. oralis (22.3%), and S. koreensis (1.6%) were discovered on the masks worn for 4–8 h. In addition, S. epidermidis (60.0%), S. aureus (6.7%) and S. oralis (13.3%) were found on the inner layer of the masks worn for more than 8 h; however, S. capitis and S. koreensis were not detected (Figure 2).
Furthermore, the contamination rates on the outer layer of the masks also increased as the mask-wearing time increased (<4 h: 0.8 × 108 CFUs; 4–8 h: 6.2 × 108 CFUs; and >8 h: 15.2 × 108 CFUs) (Figure 2). When the mask-wearing time was less than 4 h, half of the bacteria found were S. oralis (50.0%). On the masks worn for 4–8 h, S. epidermidis (41.2%), S. aureus (9.2%), S. capitis (2.7%), S. oralis (9.2%), and S. koreensis (2.7%) were discovered. Additionally, S. epidermidis (60.0%) and S. aureus (10.0%) were found on the outer layer of the masks worn for more than 8 h; however, S. capitis, S. oralis and S. koreensis were not detected (Figure 2).
Intraoral examinations were conducted to evaluate the factors affecting mask contamination, and the correlations between these factors and the mask contamination rate on the inner layer (in CFUs) were analyzed (Table 1). The participants had 27.3 ±1.6 teeth on average, and 15.1% of the participants had semi-erupted teeth (1.9 ±0.8). The participants who had undergone preventive dental treatment, such as coating with a dental sealant, constituted 58.5% of the total sample. Among them, the average number of teeth covered with a sealant was 3.9 ±2.7. In addition, 62.3% of the participants had been provided with preservation and/or prosthetic treatment, such as resin fillings or gold crowns (6.6 ±3.8 teeth on average). Furthermore, 56.6% of the participants had caries. Among them, the average number of teeth with dental caries was 3.0 ±2.3, and 9.4% and 11.3% of the participants had attritions (1.8 ±1.3) and abrasions (8.0 ±6.7), respectively. It was also observed that 11.3% of the participants had PD > 4 mm (4.9 ±3.2 mm; r = −0.109), 56.6% experienced bleeding (bleeding rate: 3.2 ±3.6%; r = 0.008) and 77.4% had calculus (calculus rate: 8.9 ±13.3%; r = −0.117). Of the total participants, 5.7% were undergoing orthodontic treatment, and 20.8% were equipped with retainers after the completion of orthodontic treatment. Table 1 shows the correlations between particular factors and the CFU count, as well as sample percentages, and the average values for teeth or the indices examined.
Saliva tests were conducted to analyze the correlations between saliva characteristics and mask contamination on the inner layer (Table 2). The unstimulated saliva secretion rate was 0.5 ±0.3 mL/min, the initial saliva pH was 6.8 ±0.3, the stimulated saliva secretion rate was 1.1 ±0.5 mL/min, and the saliva buffering capacity was 9.2 ±2.0.
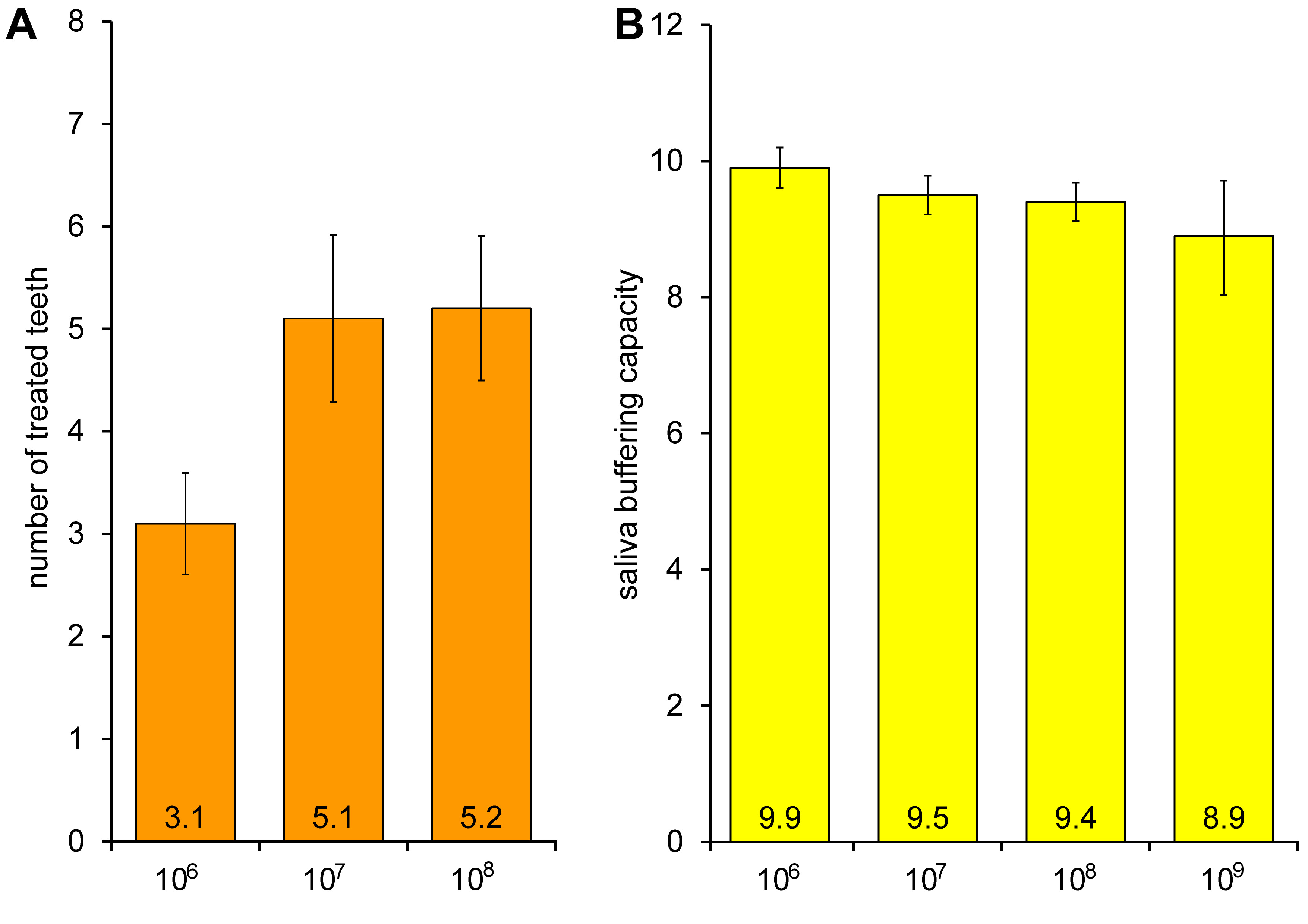
All factors were graphically analyzed. The inner layer contamination rate increased (from 3.1 × 106 to 5.2 × 108 CFUs) as the number of treated teeth increased, but the differences were not statistically significant (Figure 3). In addition, as the saliva buffering capacity decreased (from 9.9 to 8.9), the contamination rate on the inner layer of the masks increased. These differences were not statistically significant, either (Figure 3).
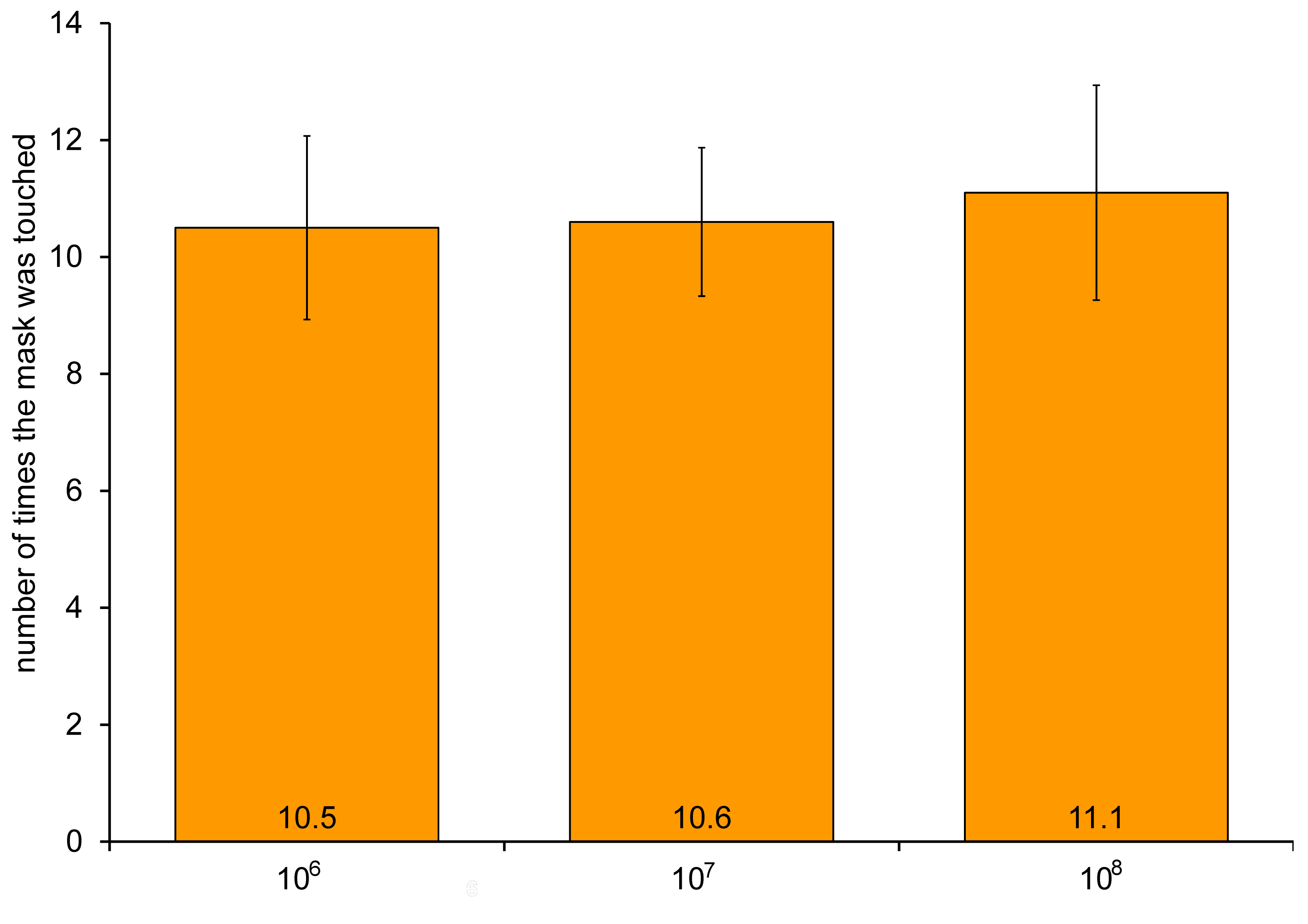
We hypothesized that there could be a correlation between the number of times the mask was touched and mask contamination on the outer layer; hence, behavioral factors were analyzed. The participants touched the mask approx. 10.7 ±9.9 times, and the outer layer contamination rate increased (from 10.5 × 106 to 11.1 × 108 CFUs) with the number of times the mask was touched. Yet, the differences were not statistically significant (Figure 4).
Discussion
The results of studies on mask contamination differ. In some articles, contamination was higher on the inner layer of masks,6 while in others, contamination was higher on the outer layer.7, 8 These varying outcomes are probably due to the environment in which the participants wore masks. The contamination outside the mask is higher in dental clinics, where aerosol-generating procedures occur.7, 8 However, under strict control over contamination conditions (e.g., in operating rooms), the contamination rate is higher inside the mask.6 Due to the generation of aerosols, air pollution in dental clinics is relatively higher than in other environments. Consequently, in the present study, the contamination rate on the outer layer of the masks was higher than on the inner layer in most samples (Figure 1).
We performed 16S rDNA sequencing to identify bacterial species. The bacteria detected on both layers were classified as the Staphylococcus and Streptococcus genera, which are Gram-positive, facultative anaerobic bacteria. They are also part of the normal human flora, typically the skin flora and, less commonly, the mucosal flora. The normal flora may be easily found on the used masks, as wearing a mask involves close contact with the human skin (Figure 1).4, 9
Some bacteria belonging to the Staphylococcus, Streptococcus and Moraxella genera were observed only on the inner layer of the masks. For example, S. salivarius is commonly found in the oral cavity,10 and S. infantis,11 S. rubneri,12 S. australis,13 S. sanguinis,14 and S. parasanguinis15 can be detected in the oral cavity and the throat. In addition, S. lugdunensis is pathogenic and can cause various infections.16 Moraxella osloensis is a commensal Gram-negative bacterium that causes invasive infections, mainly in immunocompromised individuals.17 However, if infectious bacteria were found inside the mask, we could not ascertain whether the mask wearer was also infected. It is necessary to conduct a future study that would assess the relationship between the bacteria found on the inner layer of masks and the participants’ medical history.
Some bacteria belonging to the Staphylococcus, Streptococcus, Enterococcus, Psychrobacillus, Bacillus, and Lactobacillus genera were observed only on the outer layer of the masks. Streptococcus mitis18 and E. faecium19 are commonly found in the human oral cavity or gastrointestinal tract. Therefore, they can be easily observed on the used masks. Staphylococcus warneri20 and S. caprae21 can cause opportunistic infectious diseases in immunocompromised individuals, but they are also found in the normal flora. Interestingly, some bacteria rarely found in humans, such as P. lasiicapitis, B. thioparans and L. plantarum, were found on the outer layer of the masks. This was probably due to the contamination of the mask through an unexpected route. Future research on whether these bacteria can be found in humans is needed.
On the inner and outer layers of the masks, 13.2% of bacteria were of the same species and strain. Thus, there is a possibility that the contamination inside the mask due to human-derived bacteria may spread to the outer layer, or the contamination outside the mask due to external air pollution may spread to the inner layer (Figure 1).
In our previous study, the CFU count on the inner layer of masks increased with the mask-wearing time.4 Similar to our previous results, the mask contamination rate increased with the increasing mask-wearing time (Figure 2). In the previous study, the mask contamination rate in mask samples was 0.35 × 108 CFUs and 0.95 × 108 CFUs after 2 h and 4 h of wearing, respectively.4 Similarly, the present study showed that the mask contamination rate on the inner layer after 4 h of wearing was 0.7 × 108 CFUs, which is within the range of the previous results.
Bacteria such as S. epidermidis and S. aureus are commonly found on the skin, and they survive relatively well even in dry environments; hence, they could survive for more than 8 h on the masks (Figure 2).9
Some bacteria, including S. capitis, S. oralis and S. koreensis, were only found on both the inner and outer layers of the masks worn for 4–8 h (Figure 2). Staphylococcus capitis is part of the normal flora of the human skin20 and can survive well in dry environments; however, it was not detected on the masks worn for more than 8 h. This might be due to the fact that the ability to withstand dry environments for a long time varies among bacterial species, even if they belong to the same group of the normal human flora. Streptococcus oralis was observed on the masks worn for more than 4 h (Figure 2). The bacterium is easily found in the oral cavity.22 The mask is not contaminated with S. oralis immediately after it is put on, but through conversation or breathing, as the mask-wearing time increases. Although S. koreensis was found on the masks worn for 4–8 h, further research is needed for accurate interpretation.
We hypothesized that oral health conditions and saliva characteristics could cause the contamination on the inner layer of the masks. Hence, intraoral examinations and saliva tests were performed. However, no correlations were found between those factors and the CFU count (Table 1, Table 2).
The contamination rate on the inner layer of the masks increased as the tooth treatment rate increased. In addition, as the saliva buffering capacity rate decreased, the contamination rate of the inner layer of the masks increased as well. However, these differences were not statistically significant (Figure 3). Reduced saliva buffering capacity and a decreased pH maintenance ability of saliva increase the possibility of oral diseases, such as dental caries.23 It was expected that poor oral health conditions might increase the contamination of the inner layer of the masks; yet, their impact was not pronounced.
Finally, it was hypothesized that the contamination rate on the outer layer of the masks would increase with the number of times the mask was touched. Indeed, the contamination rates increased, but the differences were not statistically significant (Figure 4), perhaps due to the complex source of contamination of the outer layer of the masks.
Due to the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, wearing a mask has become more important. Several previous studies revealed that saliva could serve for the detection of SARS-CoV-2.24, 25, 26 Thus, wearing a mask is essential for protecting not only mask wearers, but also individuals around them from the risk of infection. Patients must take off their masks to receive dental treatment; hence, it is essential for clinicians to wear masks. According to the infection control guidelines, the sterilization of equipment is essential. However, the masks worn by clinicians do not undergo a separate sterilization process. This suggests that mask contamination through an unexpected route may cause secondary infection. Therefore, it is recommended that masks that have undergone a photo-sterilization process27 with the use of ultraviolet (UV) irradiation,28 which is widely used in dentistry and does not cause damage to the mask or filter, be used.
Limitations
Anaerobic bacteria were not investigated in this experiment. Besides, the individual respiratory rates varied among the participants.
Conclusions
The contamination rates were higher on the outer layer than on the inner one. Furthermore, the CFU count on the inner and outer layers of the masks increased with the mask-wearing time. Staphylococcus epidermidis, S. aureus, S. capitis, S. oralis, and S. koreensis were found on the used masks. Oral health conditions may have affected the contamination of the inner layer, but their influence was not significant. The number of times the mask was touched may have affected the contamination of the outer layer; yet, this influence was insignificant, either.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Ulsan College, South Korea (No. 1044363-A-2022-002). All participants provided written informed consent.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.