Abstract
Background. With the recent use and development of nanomaterials, silver nanoparticles (AgNPs) are gaining much attention as a promising antibacterial agent for use in caries prevention.
Objectives. This study aimed to biosynthesize AgNPs using chamomile extract as a reducing agent and to investigate its inhibitory effect against Streptococcus mutans (S. mutans) dental bacteria.
Material and methods. Chamomile extract was prepared by sonication and added dropwise to silver nitrate (1mM) solution to synthesize AgNPs. Its formation was confirmed spectrophotometrically, and its size was determined. The disc diffusion method was used to test the antibacterial activity of the biosynthesized AgNPs against S. mutans. Also, its minimum inhibitory concentration (MIC) was assessed.
Results. The spectrum of biosynthesized AgNPs showed a maximum peak at 454 nm, and the peak area increased with increasing time. The mean AgNP size was 41 nm. The inhibition zone diameter recorded for AgNPs against S. mutans was 10 mm, while the MIC was 280 µg/ml.
Conclusions. AgNPs biosynthesized using chamomile extract were proven to exert good antibacterial activity against cariogenic S. mutans. Using chamomile extract as a reducing agent can provide a rapid, affordable, and eco-friendly approach for AgNP production, which could be incorporated into various dental vehicles for dental caries prevention.
Keywords: silver nanoparticles, Streptococcus mutans, chamomile, antibacterial, dental caries
Introduction
Dental caries is the most common oral disease in childhood. The World Health Organization (WHO) reported that dental caries affects 60–90% of school-aged children, negatively affecting the quality of life of children and their families. Caries can frequently result in extreme discomfort and growth retardation and have negative effects on body weight and height in children.1, 2
Dental caries is a multifactorial, dynamic, biofilm-mediated, sugar-driven disease that causes demineralization of the dental hard tissues.3
Streptococcus mutans (S. mutans) are Gram-positive bacteria commonly found in human dental plaques. S. mutans is the principal bacteria involved in the onset of dental caries because of its acidogenic and aciduric properties, which lead to colonization of the tooth surface, production of dental plaque, and demineralization of hard dental tissue.4, 5 Since its discovery as the causative agent of dental caries, this bacterium has been studied as a potential target for disease prevention through antimicrobial drugs and the development of a vaccine.6
Silver has been used as an antimicrobial agent for over a century due to its wide range, low toxicity, and absence of bacterial resistance. Silver nitrate is one of the most common silver salts exhibiting antimicrobial properties and has been widely used as a caries preventive agent for permanent molars, a cavity sterilizing agent, and a dentine desensitizer to reduce the occurrence of caries in deciduous dentition.7
Nanotechnology is viewed as a pioneering and realistic research field due to the growing desire for advances in diagnosis and treatment approaches.8
Using silver nanoparticles (AgNPs) to prevent and treat dental caries by inhibiting biofilm development and regulating the demineralization and remineralization balance is a promising approach for tooth decay prevention and treatment.9
AgNPs have 25 times greater antibacterial activity than chlorhexidine and have antiviral and antifungal activity. In different preparations, studies have indicated their use shows promising results for treating early dental caries.10 Moreover, adhesive systems and composite resins can benefit from the incorporation of AgNPs, as they can prevent secondary caries by exerting significant antibacterial effects at low concentrations.11
Many years ago, various chemical and physical technologies, such as laser ablation, lithography, chemical vapor deposition, sol-gel technology, and electro-deposition, were used to synthesize nanoparticles (NPs). However, these approaches are expensive and have also been documented to yield substances that are potentially dangerous to individuals and the environment.12, 13
The use of plant extract in the synthesis of NPs is particularly popular and is gaining much attention globally. Indeed, the plant-based synthesis of NPs is simple, inexpensive, environmentally benign, and less harmful for human therapy.14, 15, 16
Chamomile is one of the most widely used medicinal plants (Matricaria recutita L.). The species is native to Europe and Asia but is found almost worldwide.17 More than 8000 tons of chamomile raw material are collected annually globally, with the primary producers being Argentina, Egypt, Poland, and Hungary.18
Chamomile has been recognized as an anti-inflammatory, antibacterial, and wound-healing promoter. It decreases plaque development and boosts gingival health with other herbal ingredients such as mouthwash or dentifrice.19, 20
Several in vitro studies evaluated the antimicrobial activity of plant-mediated AgNPs against oral pathogens. AgNPs derived from the leaves of Justicia glauca demonstrated antimicrobial activity against S. mutans, Staphylococcus aureus (S. aureus), and Lactobacillus acidophilus (L. acidophilus).21 Additionally, plant extracts of Azadirachta indica, Ficus bengalensis, and Salvadora persica demonstrated antibacterial activity against L. acidophilus, Lactococcus lactis (L. lactis), and S. mutans.22 In another study, the biogenic AgNPs derived from Gum Arabic showed antimicrobial activity against S. mutans.23
In light of combining the therapeutic efficiency of AgNPs and the medicinal potential of chamomile extract for promising use in the prevention of dental caries, the purpose of this study was to biosynthesize AgNPs using chamomile extract as a reducing agent and to investigate the inhibitory effect of biosynthesized NPs against S. mutans dental bacteria.
Material and methods
Plant material and extract preparation
Chamomile flower extract solution was prepared by weighing 5 g of chamomile flowers in 50 ml of deionized water, and the mixture was sonicated for two hours at 30°C using an ultrasonic bath at 40000 Hz. The extract was then stored in the refrigerator until use.24
Preparation and characterization of AgNPs
For the preparation of the AgNP solution, 5 ml of the chamomile extract was added dropwise to 50 ml of silver nitrate (1mM) while stirring using a hot plate and magnetic stirrer set at 50°C for 30 minutes.25 Silver nitrate used in the experiment was obtained from Sigma-Aldrich. A laser diffractometer, the Zeta Sizer Nano-series, calculated particle size distribution (Nano ZS).26
In vitro antibacterial properties of AgNPs
Kirby–Bauer disk diffusion method
(determination of the zone of inhibition)
Bauer and Kirby’s disk diffusion mechanism calculated the antibacterial activity of AgNPs (0.05 mg/ml) and chamomile extract (100 mg/ml). In this experiment, paper filter discs (1 cm) were soaked in an AgNP solution. Every bacterium’s 0.1 ml culture suspension, modified to 108 CFU /ml, was separately seeded into Muller-Hinton agar medium (Merck) and then poured into Petri dishes. At the center of each of these plates, one paper disc from each treatment was aseptically placed, and plates were incubated for 24 hours at 37°C. For each treatment, three duplicate plates were used. Bacterial growth inhibition zones were measured around each disc and recorded in millimeters.27 Negative control discs were prepared using dimethyl sulfoxide (DMSO), while positive control discs used Ampicillin as a standard antimicrobial agent. The experiments were repeated three times, and the mean data were presented.
Serial dilution method (determination of the minimum inhibitory concentration (MIC))
To assess the antibacterial potential of the biosynthesized AgNPs, MIC was estimated. A series of AgNP concentrations were used to estimate the MIC values and confirm their antibacterial potentials against S. mutans ATCC 25175 using the agar dilution method. S. mutans stationary phase culture was prepared at 37°C and used to inoculate a fresh 5 ml culture to an OD600 of 0.05. The 5 ml culture was then incubated at 37°C until an OD600 of 0.1 was obtained, from which standardized suspensions of bacteria were prepared for a final cell density of 6 × 105 colony-forming units (CFU)/ml. Different amounts from the tested samples (0–640µl/l) were prepared and combined with 5.0 ml of the standardized bacterial suspension from the measured sample, then applied to the plates and incubated at 37°C for 24 hours. For each sample concentration, CFU were counted and compared with the growth of untreated controls.28
Results
AgNP formation and characterization
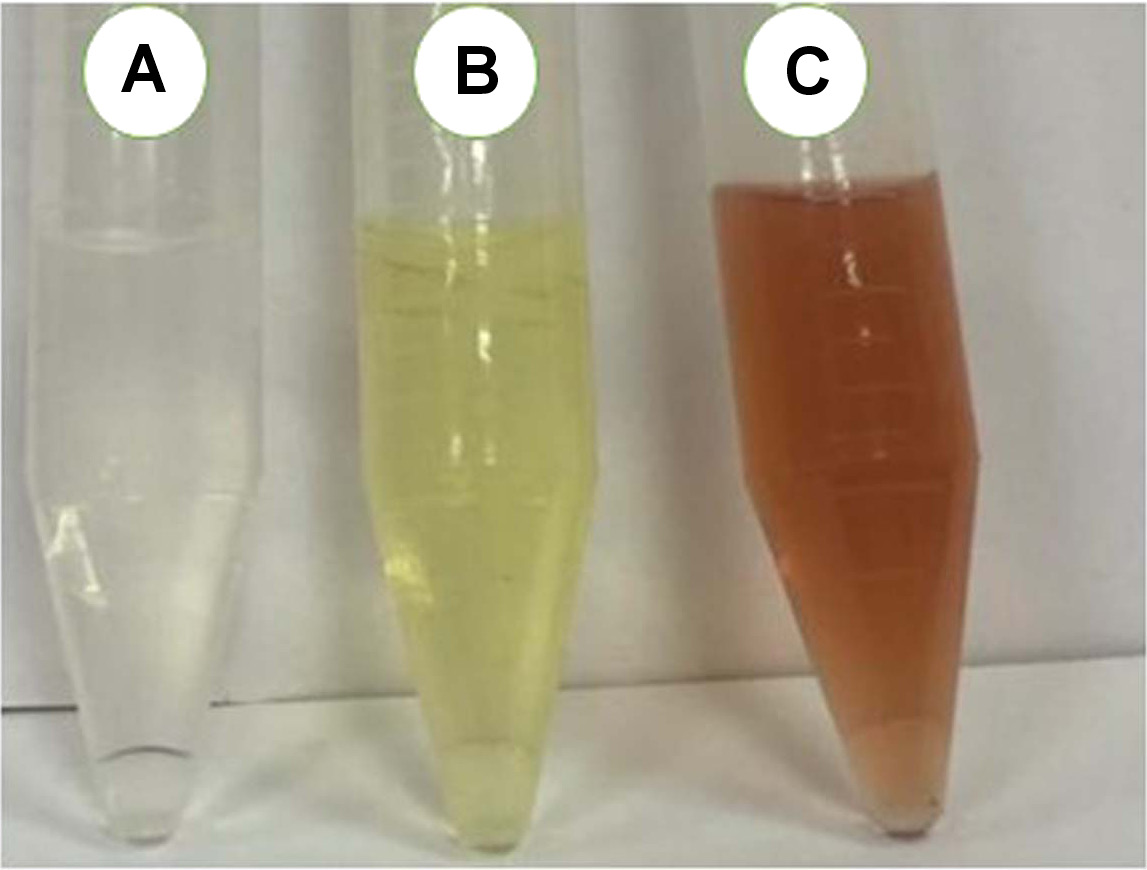
The formation of AgNPs was observed visually as the solution color changed from a clear to a light brown color (Figure 1). This color change was the first evidence of silver phyto-reduction and AgNP formation.29, 30
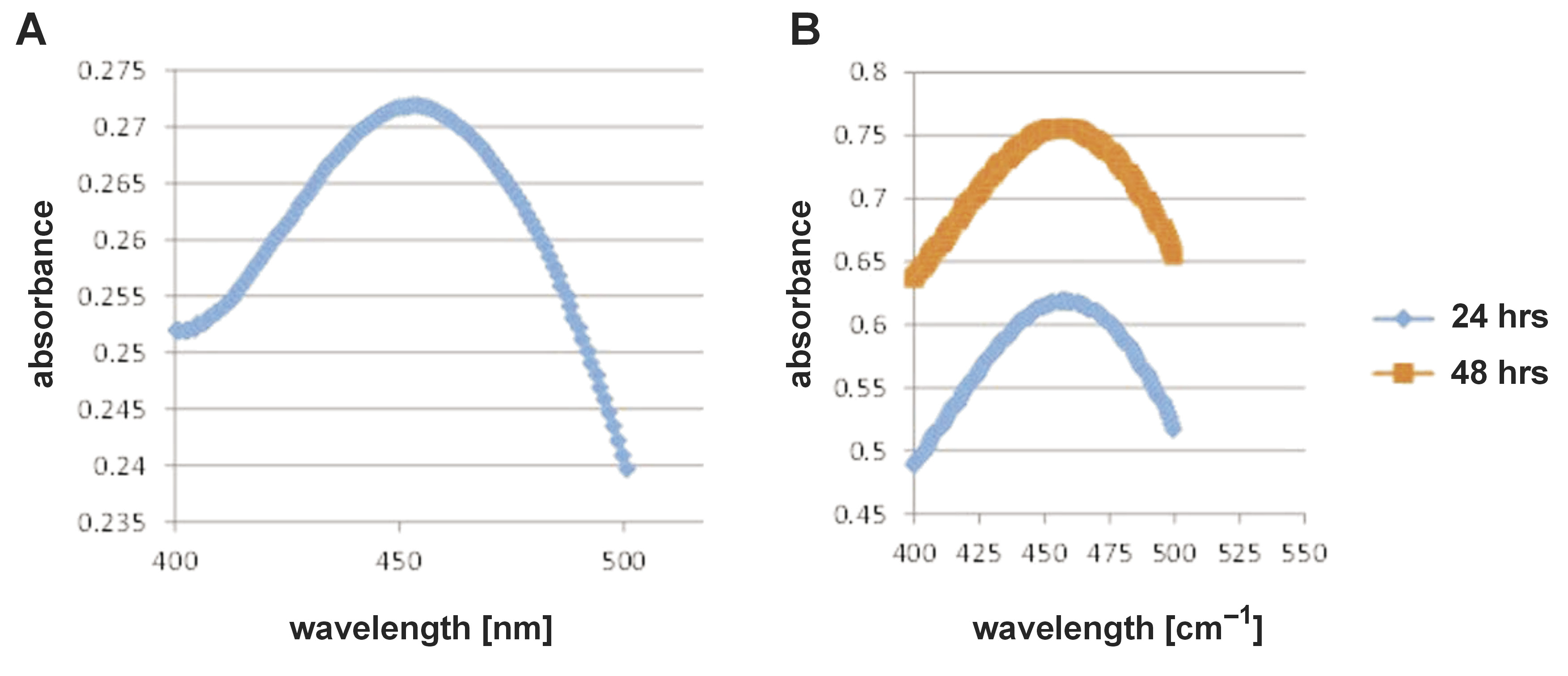
In the present work, the ultraviolet (UV)-visible spectrum of AgNP biosynthesis using chamomile extract (Figure 2) showed a maximum peak at 454 nm, corresponding to the plasmon absorbance of AgNPs.
The stability of biosynthesized AgNPs was observed over 48 hours (Figure 2), and the spectra showed that the peak area increased with increasing time.
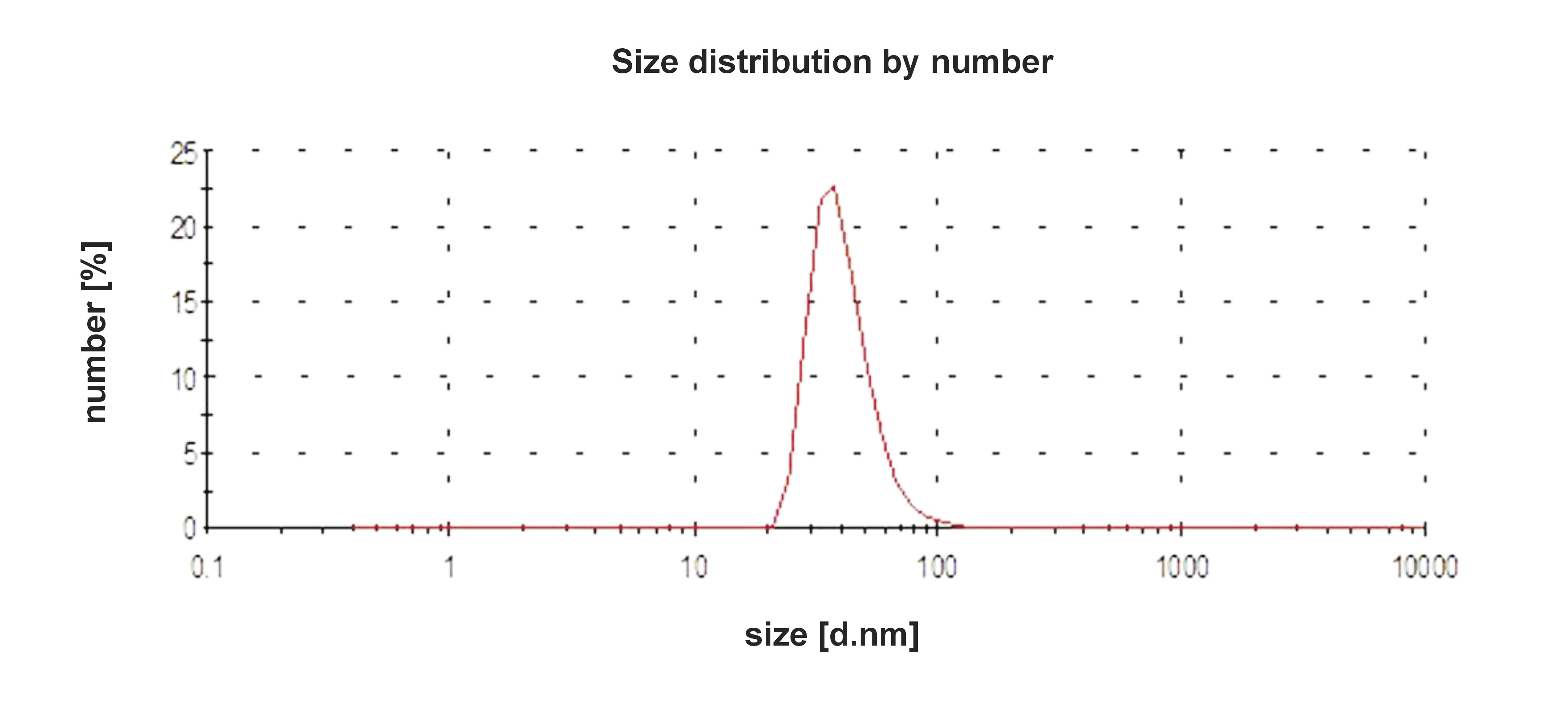
The average size of the biosynthesized AgNPs was 41 nm. The quality of the biosynthesized AgNPs was confirmed by the single peak obtained (Figure 3). The data also revealed that the AgNPs biosynthesized using chamomile extract have hydrodynamic diameters, Z-average (nm) 76.50, and a polydispersity index value of 0.205.
Antibacterial activity of AgNPs against S. mutans
In the present study, the AgNPs biosynthesized using chamomile extract exhibited moderate antimicrobial activity against S. mutans, as shown by the inhibition zone’s diameter. The mean inhibition zone diameter recorded for AgNPs against S. mutans was 10 mm (Table 1).
Minimum inhibitory concentration of AgNPs against S. mutans
In the present work, the MIC of biosynthesized AgNPs against S. mutans was 56 ±4.1 µg/ml (Table 1).
Discussion
Eliminating the S. mutans bacterial load from the oral cavity is one of the most critical biological targets for dental caries prevention.31
Several studies evaluated the antimicrobial effect of nanosilver on S. mutans and showed that it had a high impact at low concentrations.32, 33, 34 Nevertheless, the antimicrobial effect of biologically synthesized AgNPs using chamomile extract against S. mutans dental bacteria has not yet been investigated. Thus, the present study aimed to biosynthesize AgNPs using chamomile extract as a reducing agent and research the inhibitory effect of biosynthesized NPs against S. mutans dental bacteria.
Green syntheses using biological molecules obtained from plant sources in the form of extracts display superiority over chemical and/or natural methods. These plant-based biological molecules go through a highly controlled assembly to make them suitable for metal NP synthesis.35 Since it is anti-inflammatory, antibacterial, widely distributed, inexpensive, easily attainable, and safe to handle, chamomile extract was chosen as a reducing agent for AgNP synthesis in this study.36
Fortunately, the formation of biosynthesized AgNPs was successfully achieved using chamomile extract as a reducing agent, which was monitored visually by the formation of a brown color in the solution. Following previous studies, the appearance of a yellowish-brown color that increased in strength during incubation indicates AgNP formation. This color change occurs due to the excitation of surface plasmon vibrations in the NPs.30, 37
The biosynthesis of AgNPs was further confirmed by UV-visible spectroscopy, one of the most important and simplest approaches for ensuring NP formation. A maximum spectrophotometric absorbance of 400–460 nm is usually indicative of AgNP formation.38 As previously reported, the spectrum of UV-vis absorption, the size, and the form of AgNP are well-known to have a very close connection.30 Therefore, the existence of a single peak in the spectrum demonstrates spherical NP biosynthesis. Furthermore, the spectrum showed no other peaks, meaning that AgNPs were the only particles formed in the solution.39
In the spectrum shown in Figure 2, the increase in the absorbance peak area with the increase in reaction time may be explained by the reduction of biomolecular silver ions.29 Based on the particle size distribution results seen in Figure 3, it is generally agreed that when the polydispersity index (an estimate of the distribution of the population of NPs) shows higher values, this usually means a broad size distribution of several AgNPs.40
To analyze or screen the in vitro antibacterial activity of an extract of a pure chemical, a variety of laboratory procedures can be applied. The disk-diffusion and broth or agar dilution procedures are the most well-known and basic approaches.41 In the present study, the disk diffusion method was used because it is a simple and sensitive method that provides categorical results that can be easily comprehended by clinicians.42
Up until now, AgNPs’ precise antimicrobial mechanism of action is uncertain. However, investigations have revealed that being nanoscale in size, AgNPs can easily penetrate the microbial cell wall/cell membranes via sulfur-containing proteins or thiol groups, damaging the microbial deoxyribonucleic acid (DNA) and eventually causing cell death.43, 44
In this study, the biosynthesized AgNPs showed an inhibition zone diameter of 10 mm and a MIC of 56 µg/ml. This inhibitory effect follows comparable studies in which AgNPs were biosynthesized from natural products.25, 45, 46, 47 Several previous studies have reported higher MIC values for AgNP inhibition against S. mutans strains, e.g., 60 ±22.36 µg/ml,48 625µg/ml,49 50 µg/mL, and 200 µg/mL.50
Based on the findings of the present work, the AgNPs biologically synthesized using an aqueous extract of chamomile flowers appear to be a potential and effective bactericidal agent against S.mutans that might be used to prevent caries.
Conclusions
The current research has shown that the AgNPs biosynthesized using an aqueous extract of chamomile flowers have good antibacterial activity against the cariogenic pathogen S. mutans. This method can help in the quick, cost-effective, and environmentally reliable synthesis of AgNPs, which can be added to various dental vehicles such as mouthwashes or toothpaste to prevent dental caries. In dentistry, there are limited commercially available products with AgNPs in their composition. Therefore, future studies should be carried out to provide additional dental vehicles containing biosynthesized AgNPs using chamomile flower extract. Their toxicity should also be evaluated before being used as human products. Moreover, well-designed clinical trials are needed to assess the effectiveness of such products in preventing dental caries.
Ethics approval and consent to participate
Not applicable.
Data availability
All data generated and/or analyzed during this study are included in the article.
Consent for publication
Not applicable.