Abstract
Background. Low-level laser therapy (LLLT) has been applied for the management of craniomaxillofacial disorders, including intraoral wounds, as well as recurrent aphthous stomatitis (RAS) lesions. However, the proper combination of laser features and tissue characteristics remains the major challenge in the realm of photobiomodulation (PBM).
Objectives. The aim of the present study was to assess the feasibility of neodymium-doped yttrium aluminum garnet (Nd:YAG) laser therapy in treating RAS lesions, and to compare 2 techniques, different with regard to the distance between the fiber tip and the ulcer.
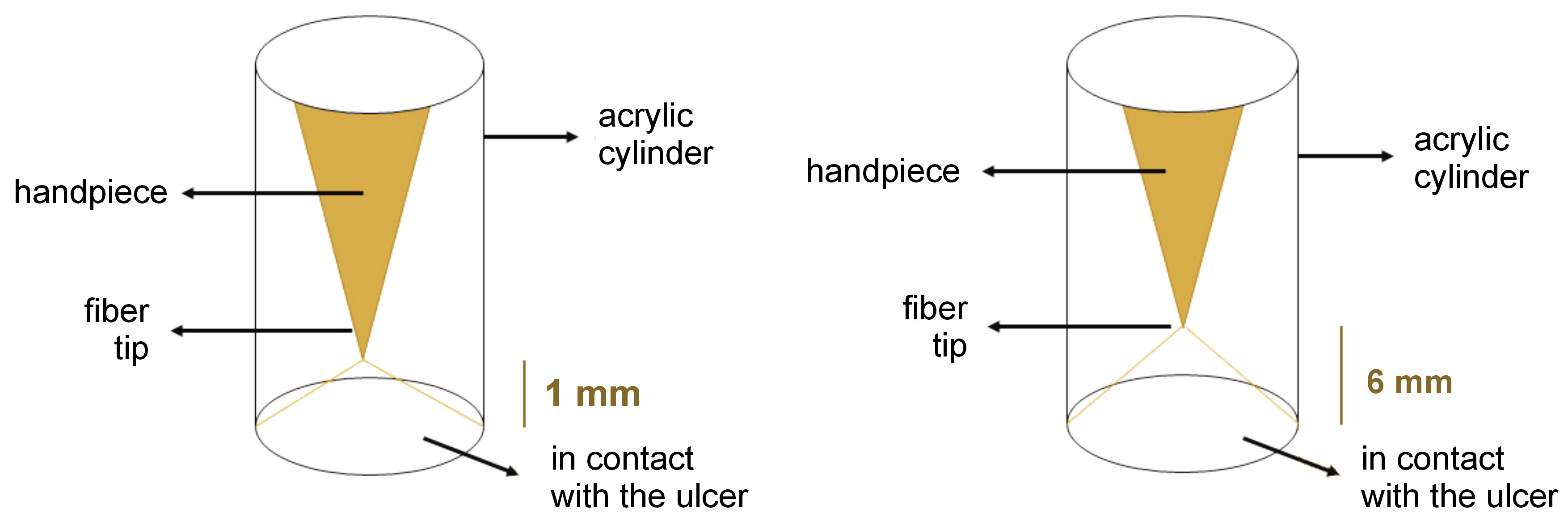
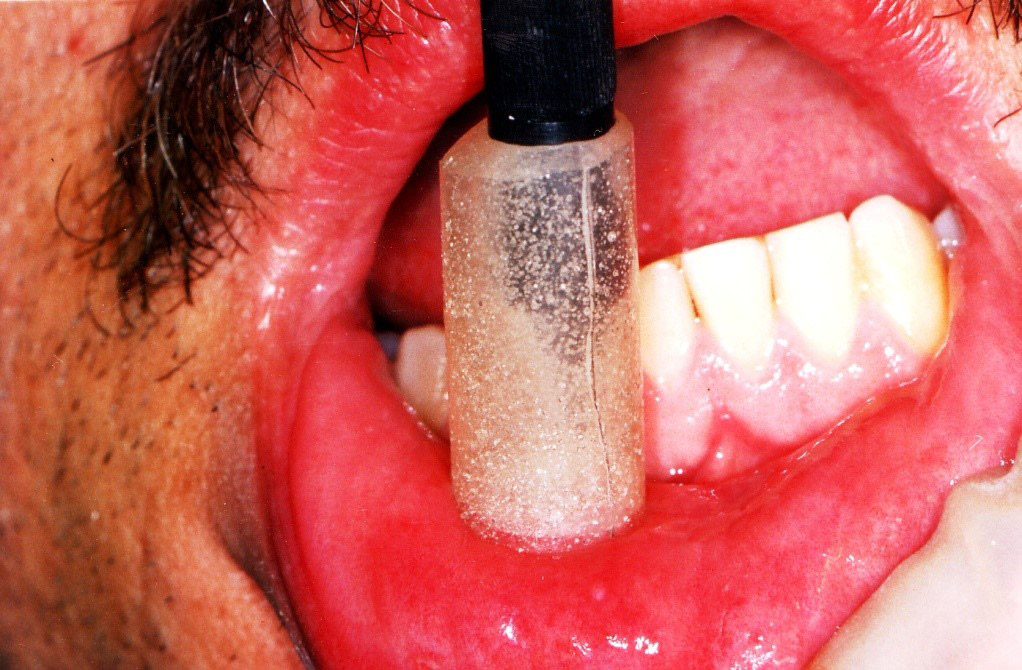
Material and methods. A total of 138 patients (94 males and 44 females) with untreated RAS were divided into 3 groups: focused laser (energy density: 48 J/cm2; power density: 0.797 W/cm2; spot size: 0.1256 cm2); defocused laser (energy density: 21 J/cm2; power density: 0.354 W/cm2; spot size: 0.2826 cm2); and placebo. In the focused group, laser irradiation was performed with the laser tip kept 1 mm away from the lesion. Acrylic cylinders were prepared to precisely fit the handpiece tip and hold it in the proper position. In the defocused group, acrylic cylinders were prepared to set the laser tip 6 mm away from the lesion to obtain defocused irradiation. Finally, in the placebo group, a routine laser therapy procedure was carried out with a helium-neon (He-Ne) red light laser. The lesion size, and pain intensity and duration were recorded.
Results. Photobiomodulation showed a significantly more efficient pain relief as compared to the placebo group (p < 0.001) and also significantly better results in decreasing pain duration (p < 0.001). Besides, the diameter of the lesions in the exposed cases decreased during the 3 consecutive days of the study, while an increase in the diameter of the lesions was noticed in the placebo group.
Conclusions. The Nd:YAG laser therapy, with the conditions and adjustments of the present study, may be successfully applied to manage RAS lesions, using either focused and defocused scanning techniques.
Keywords: lasers, aphthous stomatitis, solid-state, low-level laser therapies, oral medicine
Introduction
Recurrent aphthous stomatitis (RAS) is the most common ulcerative oral disorder, with recurring ulcers limited to the oral mucosa as the sole sign of the disease. Recurrent aphthous stomatitis is divided into 3 categories based on its clinical characteristics: minor ulcers; major ulcers; and herpetiform ulcers. Minor ulcers account for 80% of RAS cases, are less than 1 cm in diameter and tend to heal without leaving scars. Major ulcers have a diameter over 1 cm and delayed healing with scars. Herpetiform ulcers are regarded as a distinct clinical entity that presents as recurrent multiple small ulcers throughout the oral mucosa. The disease often appears for the first time during the 2nd decade of life. Pain and discomfort occur for at least 5 days in minor lesions to a maximum of 42 days in major aphthous ulcers.1
Recurrent aphthous stomatitis is currently treated in an empirical and non-defined manner. Local and symptomatic treatment modalities are the accepted procedures in simple cases of RAS. In more severe cases, topical therapies are still efficacious in accelerating the healing process, but fail to affect the attack intervals. Systemic immunomodulating factors, namely colchicine, pentoxifylline, prednisolone, dapsone, levamisole, thalidomide, azathioprine, methotrexate, cyclosporine A, interferon alpha, and tumor necrosis factor (TNF) antagonists, have been proven beneficial in resistant cases of major RAS, and have been indicated in aphthosis with systemic manifestations.2 All therapies are palliative, and none results in permanent remission. Also, many medicaments, including thalidomide, dapsone, and colchicine, have shown various adverse reactions. In addition, corticosteroids have augmented the list of the local and systemic iatrogenic diseases with which both the clinician and the patient must deal.3, 4, 5
On the other hand, the metabolic, immunologic, analgesic, regenerative, and stimulative effects of photobiomodulation (PBM) are well known to the healthcare profession.6, 7, 8 Much time has passed since it was discovered that therapeutic laser irradiation accelerated healing and decreased inflammation. Photobiomodulation has been used to manage craniomaxillofacial disorders, including intraoral wounds and RAS lesions. Helium-neon (He-Ne) (in the form of both gas and diode), therapeutic (defocused) carbon dioxide (CO2), gallium arsenide (GaAs), erbium family, and therapeutic (defocused) neodymium-doped yttrium aluminum garnet (Nd:YAG) lasers have been used to treat RAS ulcers.9, 10, 11, 12, 13, 14, 15
The exact mechanism behind the impact of PBM is still unknown. However, several theories have been proposed. The adenosine triphosphate (ATP) hypothesis states that the cytochromes of mitochondria absorb light and convert it to energy.16 Next, the cell is bioactivated and uses the energy to activate its intracellular structures. Meanwhile, the hyperpolarization following laser therapy confines neurotransmission and decreases the pain level.17
As Tunér and Hode previously stated in the preface of their book, things that require further clarification are the ideal doses, intensities, intervals of treatment, and other details in this delicate procedure.18 The proper combination of laser features and tissue characteristics remains the major challenge in PBM. Thus, the current study aimed to assessed the feasibility of the therapeutic Nd:YAG laser treatment of RAS lesions, and to compare defocused and focused scanning.
Material and methods
A total of 243 patients were examined at the Dental School of Tehran University of Medical Sciences, Iran, over 2 years, and 138 patients (94 males and 44 females) met the inclusion criteria of this randomized clinical trial (ethical code: 3316). Sampling was accomplished using permuted blocks. The patients completed a questionnaire about their health condition and medical history. Only those without a systemic condition were considered for the study. Patients with untreated RAS developed within 48 h before the referral were also considered. In contrast, patients who had started taking medication, including mouthwashes, and patients with ulcerative colitis, Reiter’s syndrome or Behçet’s syndrome, herpetiform aphthous ulcers, and resistant RAS (non-responsive to treatment within the 3 weeks of the study), were excluded. Written informed consent was obtained from all patients after providing them with thorough instructions about the treatment procedure. Demographic data and examination outcomes were recorded for every patient. Study cases were then divided into 3 treatment groups: focused laser (F; n = 39), defocused laser (D; n = 46) and placebo (P; n = 28). Photobiomodulation was performed based on the parameters mentioned in Table 1.
F: Focused laser group
The optical fiber was used focally, meaning the tip approached the lesion until a well-localized spot was obtained without touching the tissue. The laser tip was then moved helically toward the periphery of the lesion and irradiation was performed, leaving an unexposed safe margin of 1 mm around the lesion. To establish a uniform pattern of the helical movement, maintain the focal distance and avoid errors due to hand tremor, alginate impressions were made from the laser handpiece, and an acrylic cylinder was fabricated for each patient. Acrylic cylinders were prepared to precisely fit the handpiece tip and hold it in the proper position (Figure 1). The Nd:YAG laser (HighTech, Ravenna, Italy) was set as follows: fiber optic = 300 µ; TEM00; T = 60 s; F = 30 Hz; P = 3 W, and E = 100 mJ. If patients reported any sense of burning throughout the procedure, the movement speed was increased.
D: Defocused laser group
The same protocol was followed, except that a defocused spot was maintained throughout the therapy. The helical movement was not necessary, though preferably used. Another acrylic cylinder was prepared to position the laser tip 6 mm away from the lesion to obtain defocused irradiation Figure 1, Figure 2). The Nd:YAG laser was set as in group F.
P: Placebo effect control
Patients were treated in the same way, but with a He-Ne red light laser (HighTech). The same movements were applied for the same duration as in the other 2 groups, but with a greater distance from the lesion (the cylinders were not used), and the laser beam was not aimed directly at the lesion.
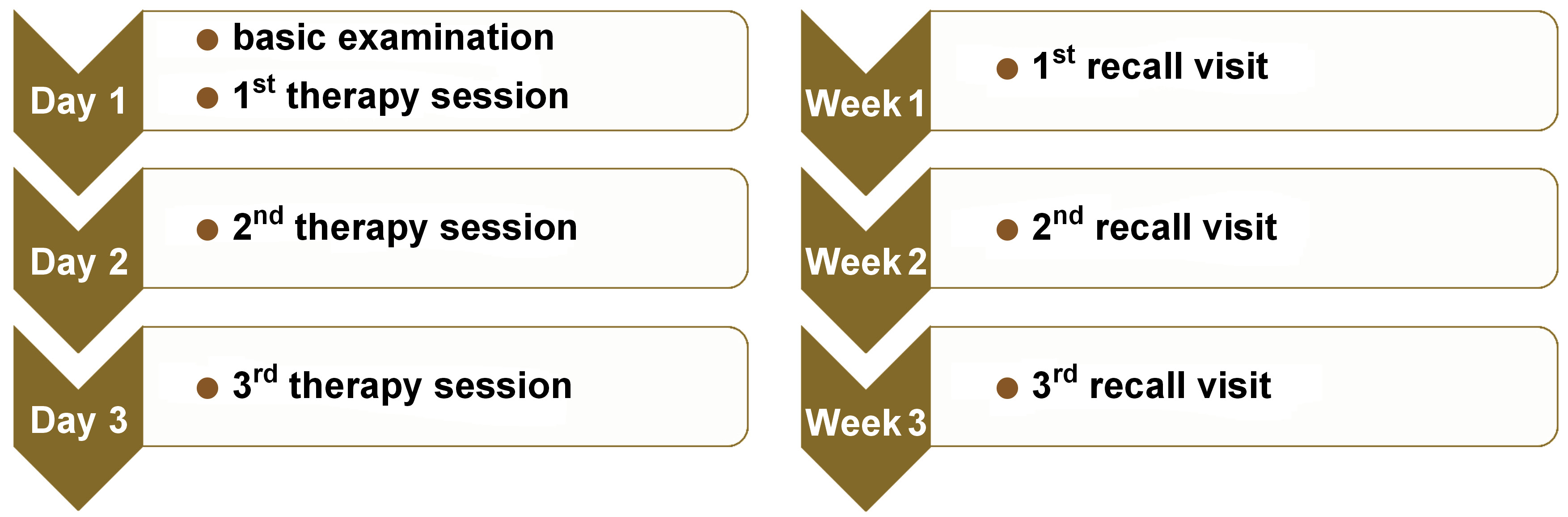
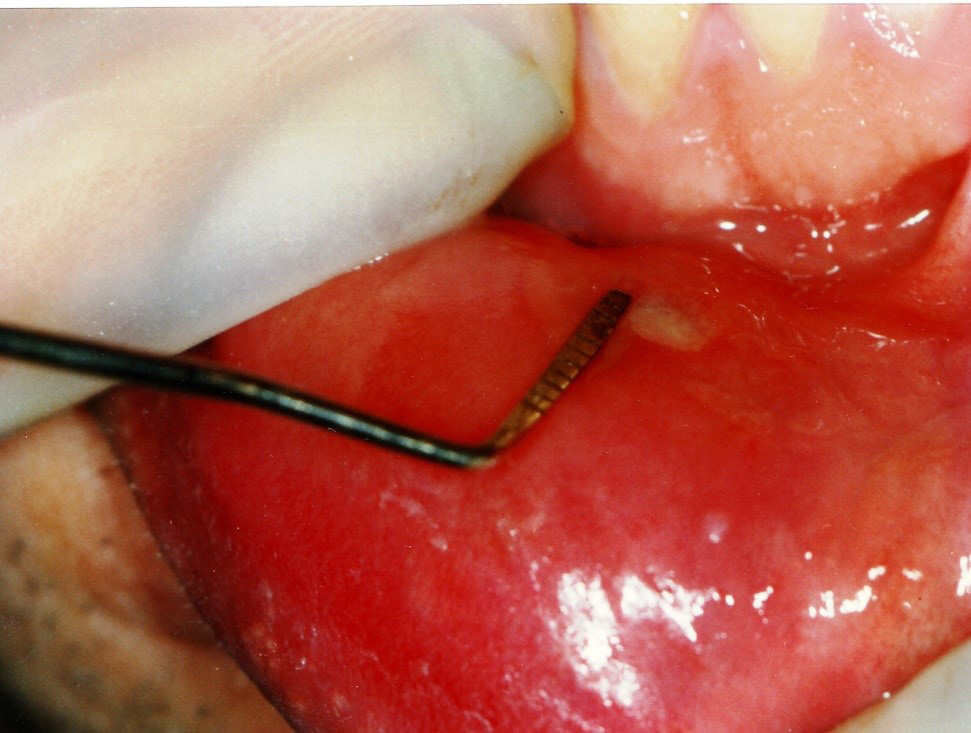
For all groups, the PBM procedure was performed on the 1st, 2nd and 3rd day. The patients were asked for recall visits on the 7th, 14th, 30th, and 60th day of treatment (Figure 3). Clinical presentations, including pain and the lesion size, were recorded at each visit. A periodontal probe was used to measure the lesion size (Figure 4). Pain intensity and duration, the recurrence intervals of both pain and lesions, and the lesion diameter were also recorded at each visit based on the consented study scales scoring from 0 to 2.
Statistical analysis
The data was classified and statistically analyzed using SPSS for Windows, v. 14.0 (SPSS Inc., Chicago, USA), the χ2 test and the one-way analysis of variance (ANOVA) (p < 0.05).
Results
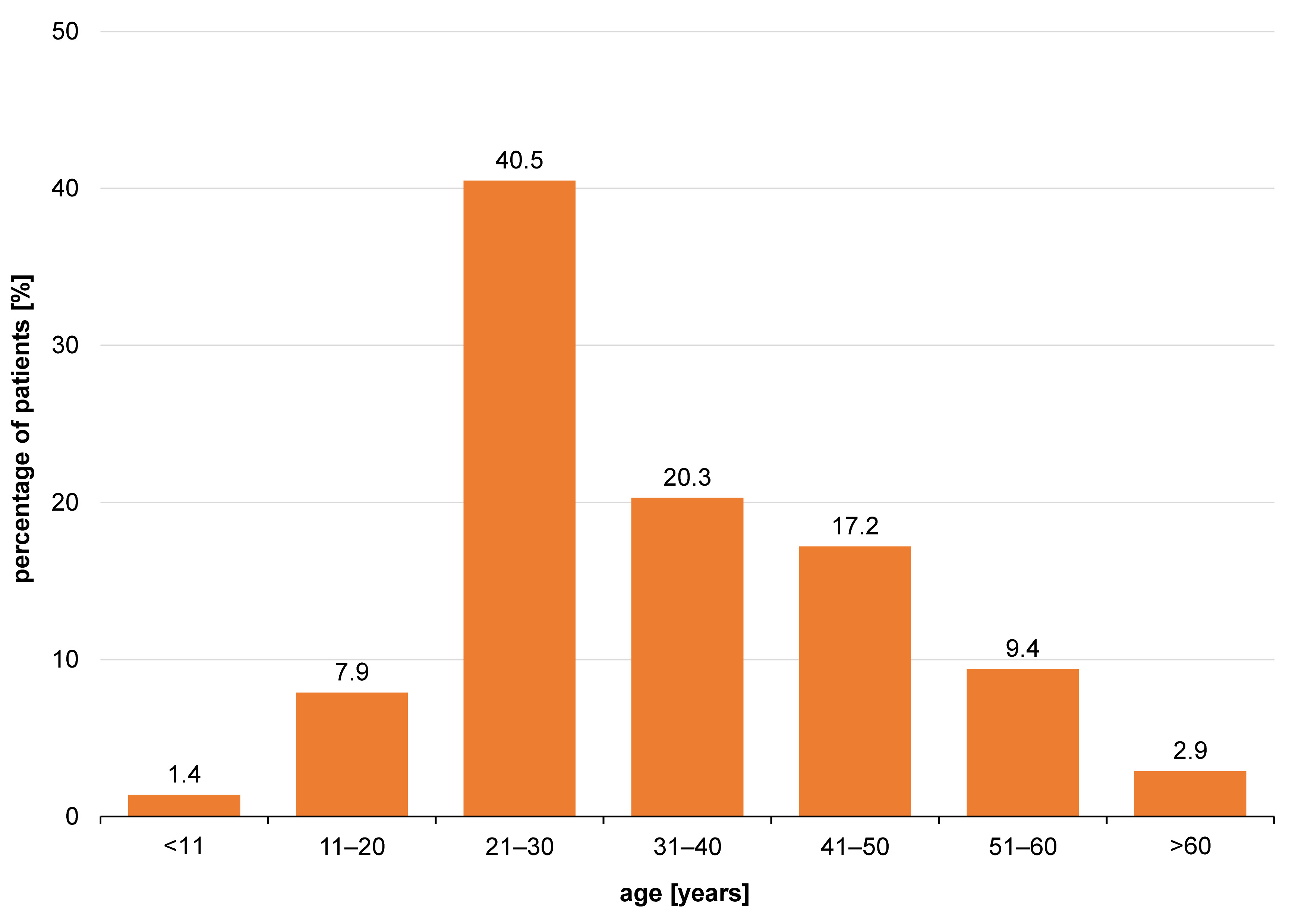
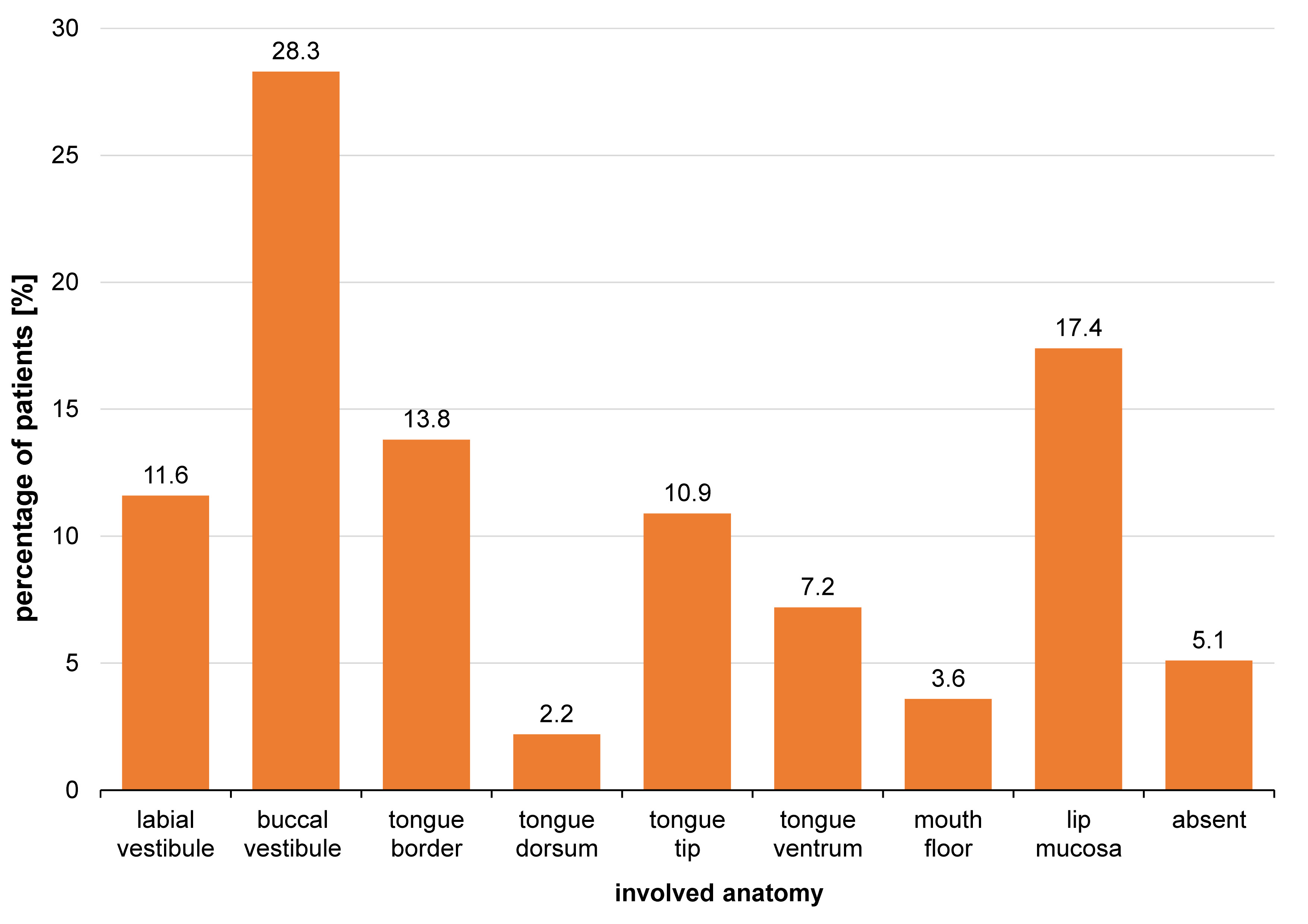
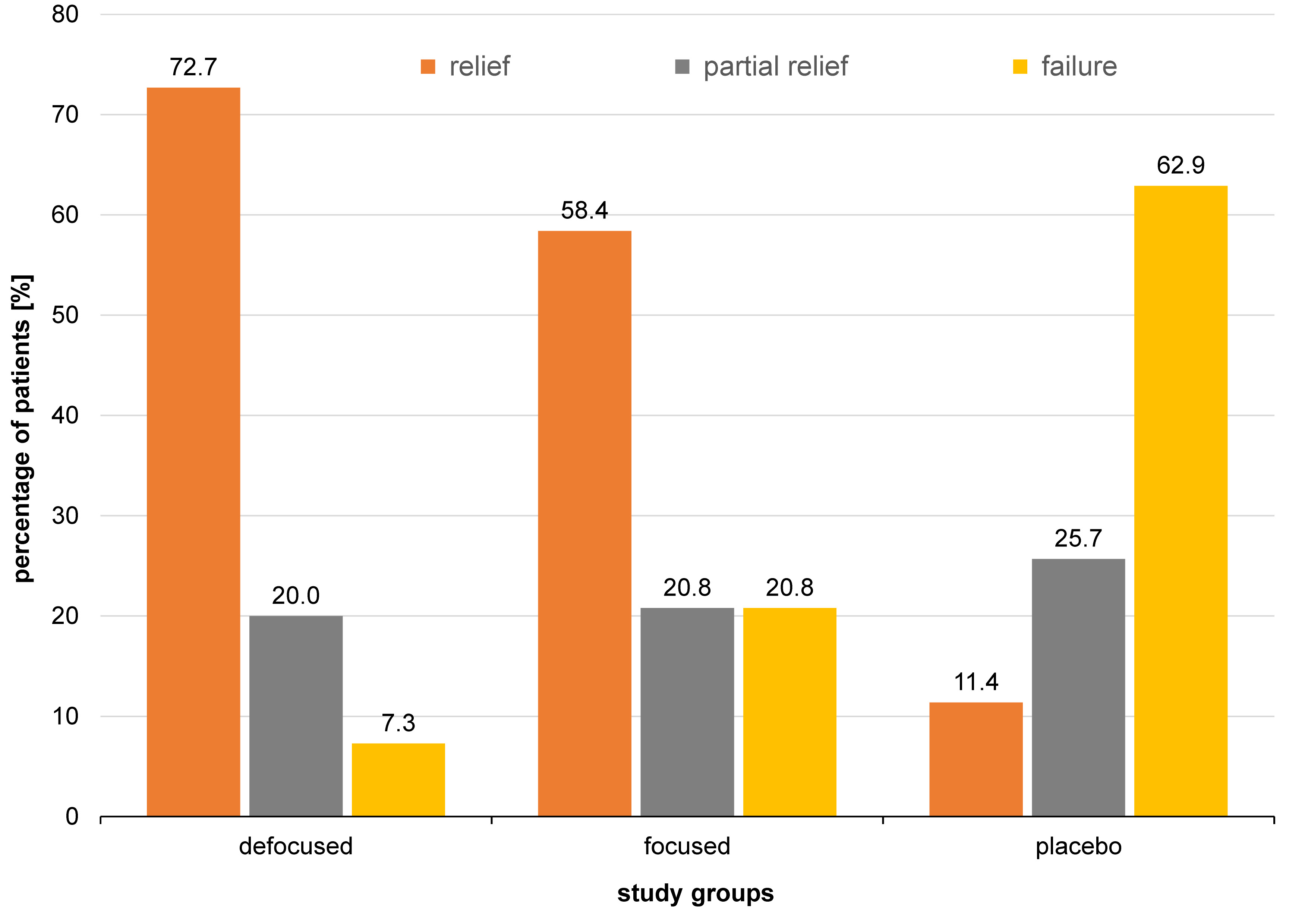
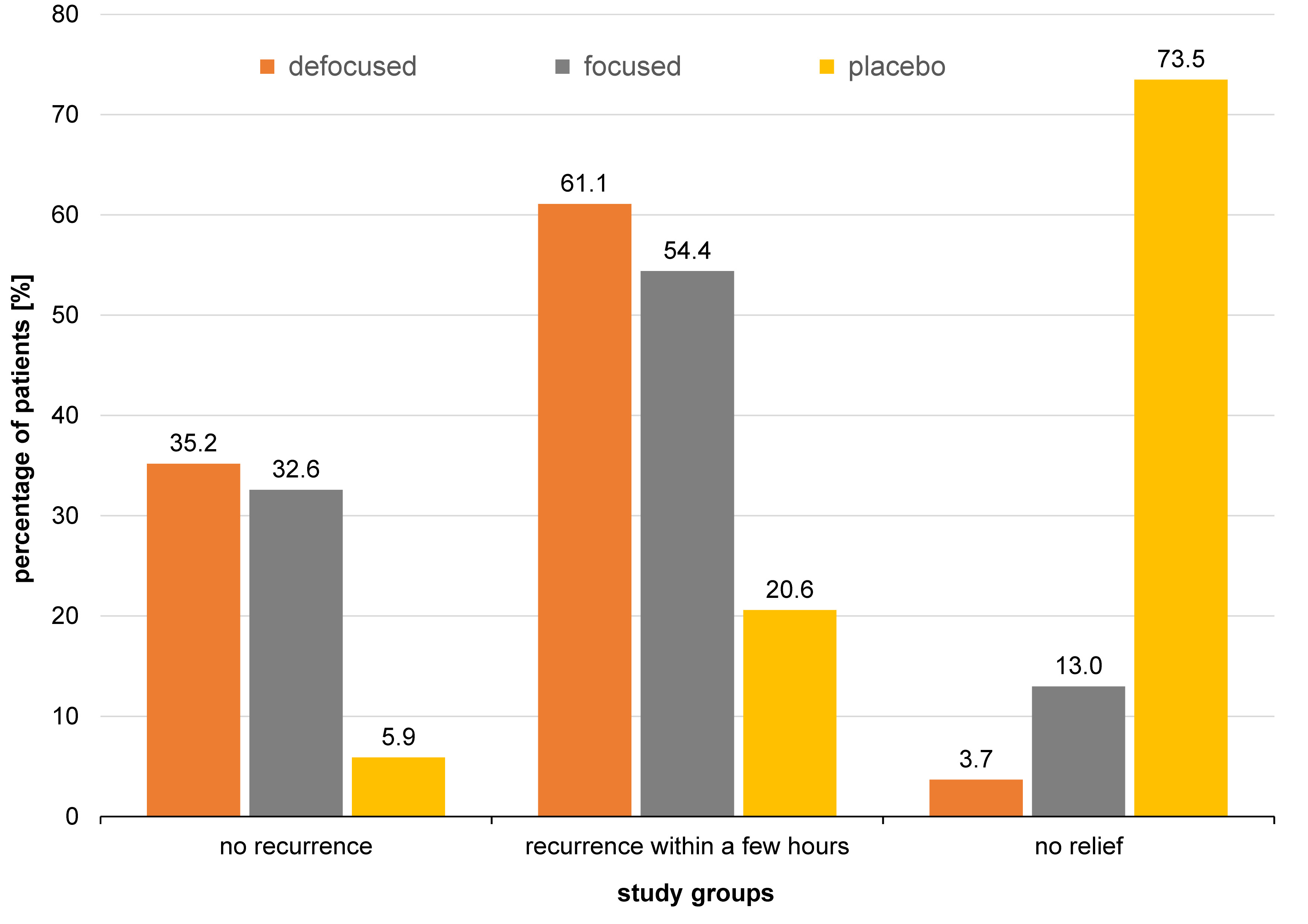
The lesions typically developed in the 3rd, 4th and 5th decade of life (Figure 5). The buccal vestibule was involved in 28.3% of cases, and the lip mucosa in 17.4%, whereas the tongue dorsum was the least involved (2.2%) (Figure 6). Photobiomodulation proved significantly more efficient in terms of pain relief (p < 0.001) (Figure 7), pain recurrence (p < 0.001) (Figure 8), healing time (p < 0.001) (Table 2), and pain duration (p < 0.001) (Table 3) in comparison with the placebo. Also, a significant difference was found between the two PBM techniques when using Tukey’s test (p < 0.05; 95% reliability), with the defocused technique being more efficient in pain relief and decreasing lesion recurrence, and the focused technique resulting in a shorter healing time. The diameter of the lesions in the exposed cases decreased during the 3 consecutive days of the study. Concurrently, an increase in the lesion diameter was noticed in the placebo group. However, the differences were not statistically significant. Total and partial pain relief was noted in 53.2% and 23.4% of males, respectively. Meanwhile, 23.4% did not report any pain relief. The results in females were 50.0%, 18.2% and 31.8%, respectively. Gender (p = 0.540), age (p = 0.430) and the lesion site (p = 0.090) had no relationship with pain relief. Also, the lesion site was not associated with the healing time (p > 0.05); however, for pain duration, the tip of the tongue showed significantly more resistance than other sites, while the lip mucosa showed the least pain resistance (95% reliability).
None of the patients required supplemental topical anesthesia along with the laser irradiation. Some of the patients in group F reported a negligible stingy feeling. No adverse reactions due to the irradiation were noted.
Most patients did not come back after the recall visits, so it was not possible to statistically analyze the recurrence. There were cases with a history of oral aphthous lesions recurring every 2 months among the study population, of which 1 returned after the recall and did not report any recurrences during 13 months. More noticeably, an 8-year-old boy with RAS recurring monthly (as reported by the parents) did not develop any lesions within 8 months of the therapy (group D) and was referred to our clinic to undergo further PBM (group D), with no lesions developing for the next 7 months. On the contrary, there were cases with recurrent lesions at the 1st recall visit at the same site or close to it.
Discussion
The main etiology of aphthous lesions is still unknown, meaning no definitive treatment option is recommended. The primary aims of routinely performed procedures are to reduce pain and the lesion size, and prevent recurrence. Photobiomodulation is one of the interventions that has been widely analyzed. In our study, PBM yielded better treatment results, with the defocused technique being more efficient in pain relief and decreasing lesion recurrence, and the focused technique resulting in a shorter healing time. Gender, age and the lesion site did not seem to affect the course of the disease or the response to the different treatment modalities (p > 0.05). However, the tip of the tongue showed significantly more pain resistance as compared to other sites, while the lip mucosa experienced the least pain duration (95% reliability).
Ahmed et al. performed a systematic review in 2020 to compare the efficacy of PBM and topical interventions used to treat patients with aphthous lesions.19 Their results were based on 330 patients included in 5 randomized controlled trials.15, 20, 21, 22, 23 They reported that PBM was a more effective treatment option than topical medications, such as triamcinolone acetonide, amlexanox, granofurin, and solcoseryl.19
The pioneering work published by Pinheiro et al. started a new era in PBM application for the clinical treatment of various disorders.24 There have been many studies on this topic since then, analyzing the impact of PBM on healing, and showing the approach to be a safe method with limited complications.25, 26 Furthermore, patients treated with this method can drink, eat and brush their teeth a few days after the procedure.10, 27
The Nd:YAG laser was utilized in the current study. However, the efficacy of other types of lasers in treating patients with intraoral wounds has also been analyzed. Indeed, the treatment protocol may impact the efficacy of PBM. However, there is limited data regarding the optimal treatment approach with Nd:YAG lasers. On the other hand, studies have been performed on other lasers to compare different exposure times and wavelengths. For instance, Rocca et al. analyzed the impact of 4 different wavelengths of PBM (i.e., 2,940 nm, 808 nm, 450 nm, and 635 nm) on treating RAS patients.28 Their results showed superior results for the 635 nm diode lasers.28 Similar to the current study, Sharon-Buller et al. reported the successful clinical application of the CO2 laser in mitigating severe pain in oral aphthosis treatment.29 The patients manifesting stress-induced, chemoradiotherapy-related and immune-associated oral aphthosis acknowledged prompt pain relief and swift recovery after PBM.29
Besides oral lesions, PBM has been applied to overcome other maxillofacial disorders. Pinheiro et al. published a thorough study on PBM for the management of maxillofacial region disorders, including temporomandibular joint (TMJ) pain, trigeminal neuralgia, muscular pain, aphthous lesions, inflammation, post- and preoperative tooth hypersensitivity, and small hemangiomas.24 Photobiomodulation has been proven to benefit the treatment of various conditions of the maxillofacial region.30, 31, 32
Recent clinical and laboratory studies have shown that the photobiological and photochemical effects of low-level lasers in injured tissues can stimulate epithelialization, vascularization and the collagen synthesis by fibroblasts. Initially, the stimulation of lymphocyte and granulocyte activity enhances the rate of necrotic tissue removal. Then, fibroblastic activity occurs, which increases the production of collagen. This is followed by the invagination of the capillaries into the regenerated tissues and the promotion of epithelialization, resulting in accelerated tissue regeneration and pain alleviation. Nonetheless, the literature on this topic lacks comparisons between the available therapeutic (biostimulation) and surgical (ablation) approaches.
Conclusions
Therapeutic Nd:YAG lasers, with the conditions and adjustments of the present study, may be successfully applied in RAS lesion management, using either focused or defocused scanning techniques.
Ethics approval and consent to participate
Ethical approval for the study was granted by the ethics committee at Tehran Beheshti University of Medical Sciences, Iran (ethical code: 3316). All participants provided written informed consent.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.