Abstract
Background. Gingivitis is frequently painless, rarely causes spontaneous bleeding and is manifested by minor clinical changes. Therefore, most patients are unaware of the disease or do not seek treatment, as it is asymptomatic. Several methods for removing microbial plaque have been proposed, including mechanical and chemical ones. Amla or Indian gooseberry is a medicinal herb; its secondary metabolites, such as phenolic acid, flavonoids and terpenoids, can be used to preferentially reduce metal ions and form nanoparticles (NPs). Green synthesis with the use of the amla seed extract is a unique approach for the production of graphene oxide (GO)-silver (Ag) nanocomposite mouthwash.
Objectives. The aim of the present study was to prepare an amla seed-mediated GO-Ag nanocomposite mouthwash, and to assess its antibacterial and anti-inflammatory efficacy in plaque-induced gingivitis.
Material and methods. The present double-blind randomized controlled trial was conducted among 30 gingivitis patients. The patients were randomly allocated into 2 groups based on the intervention: group A (n = 15; nanocomposite mouthwash); and group B – control (n = 15; 0.2% chlorhexidine (CHX) mouthwash). Clinical parameters, including the plaque index (PI), the gingival index (GI), a microbiological parameter – colony forming units (CFUs), and a biochemical parameter – the C-reactive protein (CRP) level in gingival crevicular fluid (GCF), were assessed at baseline and at 15 days.
Results. The study results showed statistically significant differences in the mean PI and GI scores, and the CRP levels in the post-intervention period as compared to baseline in both groups. After the intervention period of 15 days, there were statistically significant differences between the 2 study groups in terms of mean PI and GI scores, and CRP levels.
Conclusions. The amla seed-mediated GO-Ag nanocomposite mouthwash efficiently reduced plaque, gingival inflammation and CFUs among patients with plaque-induced gingivitis, but was not equivalent to the CHX mouthwash.
Keywords: graphene oxide, nanoparticle, silver
Introduction
Dental caries and gingivitis have been identified as the most common oral diseases across the globe. Gingivitis is an inflammatory condition characterized by gingival redness, edema and a lack of periodontal attachment loss, and is typically caused by dental biofilm accumulation.1 Furthermore, it is often painless, rarely causes spontaneous bleeding and is manifested by minor clinical changes. Therefore, most patients are unaware of the disease or do not seek treatment, as it is asymptomatic. Gingivitis can be plaque-induced or non-plaque-induced. However, in children and adults, plaque-induced gingivitis is considered the most prevalent form of periodontal disease. Plaque-induced gingivitis is defined as an inflammatory response of gingival tissues, resulting from bacterial plaque accumulation at and below the gingival margin.2 If left untreated, gingivitis can proceed to periodontitis, which involves the loss of periodontal attachment and the supporting alveolar bone, eventually leading to tooth loss, which is closely related to a negative impact on quality of life.3
In comparison with periodontitis, plaque-induced gingivitis is unique in that the tissue changes are completely reversible if biofilm accumulation is controlled. Several methods for removing microbial plaque have been proposed, including mechanical and chemical ones. According to data, mechanical plaque control does not appear to be 100% effective in certain conditions. As a result, chemical plaque control has received much attention. Chemical plaque control agents have antimicrobial, anti-plaque or anti-gingivitis properties. Chlorhexidine gluconate (CHX) and essential oils are the active chemical components in widely prescribed mouthwashes. Chlorhexidine gluconate is still considered the gold standard agent among various commercially available mouthwashes.4 Depending on the concentration, CHX acts as a bacteriostatic or bactericidal agent. The long-term use of CHX-containing drugs, despite their effectiveness in lowering the microorganism levels in the oral cavity, is associated with local side effects, such as impaired taste, tooth staining, increased supragingival calculus production, and intermittent inflammation and desquamation of mucous membranes.5
Herbal compounds have been included as ingredients in oral care products for a long time, especially in South Asian nations. Sanguinary, clove, propolis, miswak, neem, and charcoal are the most common herbal compounds included in toothpastes and mouthrinses.6 Natural products derived from medicinal plants have proven a rich supply of physiologically active molecules, with many serving as the foundation for the development of new pharmaceutical chemicals. In Indian medicine, Phyllanthus emblica or Emblica officinalis, commonly called amla or Indian gooseberry, is a valuable medicinal plant. It contains vitamin C, polyphenols, flavonols and tannins, and has antibacterial, antifungal, antiviral, antioxidant, and wound healing properties and other pharmacological effects.7 Several clinical trials on amla have found that it efficiently inhibits the formation of bacterial plaque, preventing the progression of gingivitis and periodontitis.8
The elimination of the biofilm matrix and resident bacteria by using nanoparticles (NPs) shows a lot of promise.9 Nanoparticles have a diameter of less than 100 nm, and the use of their unique properties to resist infection has expanded dramatically in the last decade. The ability of NPs to inhibit the formation of biofilms within the oral cavity, as a result of their biocidal, anti-adhesive and delivery capacities, is currently being closely examined. Among all NPs, silver nanoparticles (AgNPs) have significant antibacterial properties.10, 11, 12, 13, 14 Bacteria are less prone to develop resistance to AgNPs than to conventional antibiotics. As a result, combining graphene oxide (GO) and AgNPs to build a nanocomposite makes it a superior antibiotic to either component alone.15 The GO–Ag bond is stable, has a high oxidative potential and is hydrophilic, which results in oxidative stress. Silver releases toxic components that have a bactericidal effect, whereas GO wraps around the bacterium.
Plant-mediated nanoparticle production has recently received much attention.16 A number of plant products, such as extracts, are used to produce metal NPs, such as gold (Au), Ag, copper (Cu), and zinc (Zn) NPs.17 Plant secondary metabolites, such as phenolic acid, flavonoids, terpenoids, and alkaloids, are abundant in crude extracts; they preferentially reduce metal ions and form NPs. Green synthesis with the use of the amla seed extract is a unique approach to the production of GO-AgNP composites.
As plaque control is the key factor for the prevention of disease progression from gingivitis to periodontitis, the formulation of a novel nanocomposite could be clinically beneficial. A preliminary study including the preparation and characterization of an amla seed-mediated GO-Ag nanocomposite found antibacterial activity against Streptococcus mutans, Lactobacillus and Candida albicans, using an in vitro cytotoxicity assay.18 Therefore, the present study aimed to prepare an amla seed-mediated GO-Ag nanocomposite mouthwash, and to assess its antibacterial and anti-inflammatory efficacy in plaque-induced gingivitis.
Material and methods
Setting and design
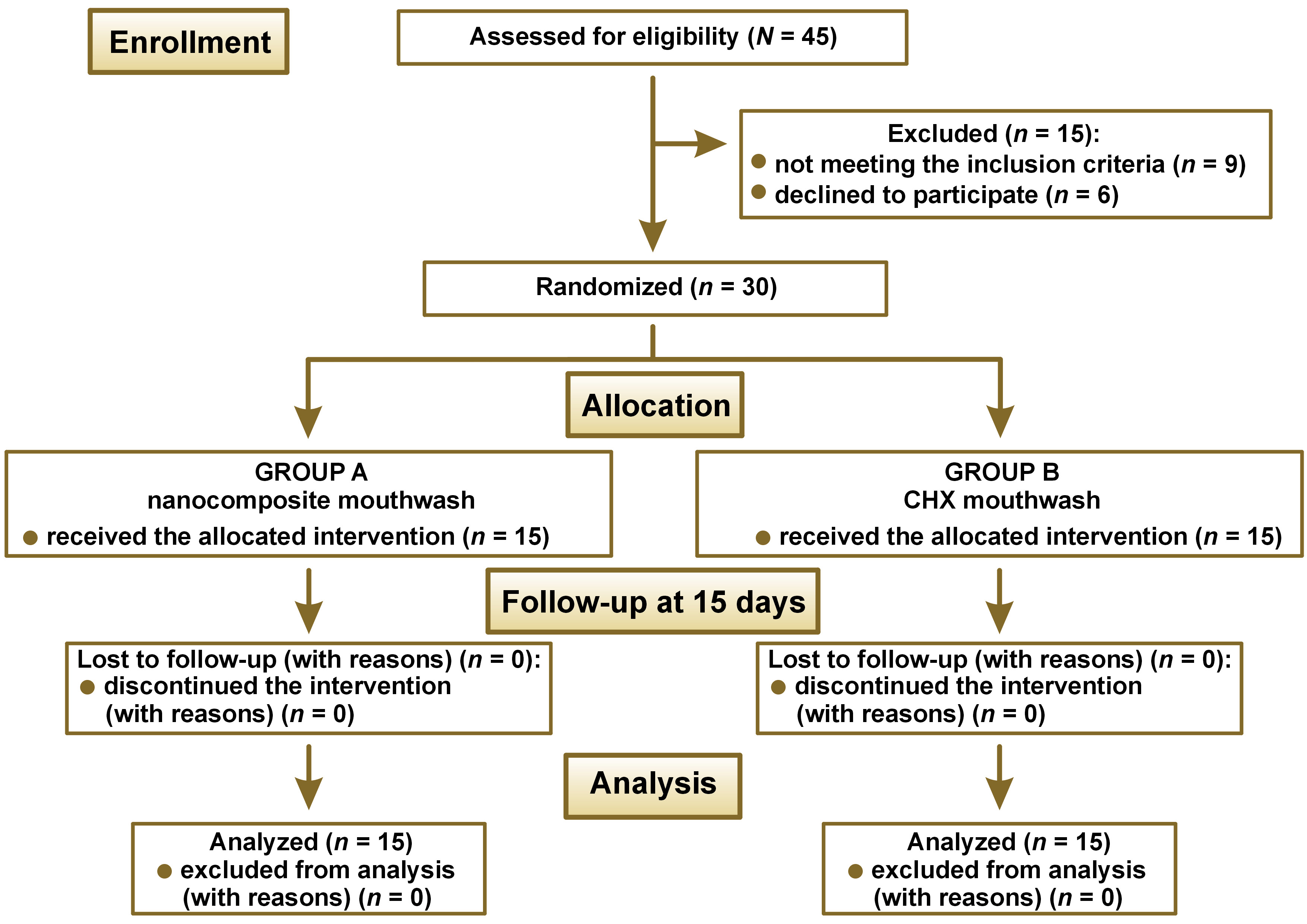
The study was a double-blind randomized controlled trial carried out at Saveetha Dental College and Hospitals, Chennai, India. The study was designed in accordance with the Declaration of Helsinki. Ethical clearance was obtained from the institutional ethics committee (IEHC/PERIO-18/2022/35), and the study participants provided written informed consent before the intervention. The study followed the CONSORT (Consolidated Standards of Reporting Trials) guidelines. The sample size was determined using the standard deviation (SD) values of a previous study19 with the G*Power software, v. 3.0 (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower), and was estimated to be 14, which was rounded to 15 for each group, and the total sample size was 30. Randomization used online random allocation software (RandomAlloc.exe., v. 1.0; https://random-allocation-software.software.informer.com/1.0), where the number of groups, the number of patients per group, and a number range were fed into the software to generate random numbers. Each patient was allotted a number, according to which they were allocated to either of the groups: group A (nanocomposite mouthwash); or group B – control (0.2% CHX mouthwash). The study design is depicted as a CONSORT flow diagram in Figure 1. The patient and the clinician were blinded. To avoid bias, the mouthwashes were given to the subjects in an amber-colored bottle.
The inclusion criteria were as follows: plaque-induced gingivitis patients; age ranging from 18 to 30 years; with a minimum of 20 teeth; no other systemic conditions; participants with plaque index (PI) and gingival index (GI) scores ≥1 in 10% of the sites; and participants willing to comply with the appointment schedule.
The exclusion criteria were as follows: generalized or localized chronic periodontitis; pregnant or lactating women; participants with any systemic condition; participants with any allergy or infectious disease; participants receiving antibiotic therapy or any medication within the past 6 months; participants using any chemical mouthwash or any other oral hygiene aids; participants wearing an orthodontic appliance or a removable partial denture; and patients who refused to be a part of the study.
Preparation of the herbal mouthwash
Preparation of the amla seed extract
Fresh amla fruits were collected, washed thoroughly and cut to obtain seeds. The seeds were finely crushed into a powder. The powdered amla seeds weighing around 25 g were carefully cleaned in distilled water for 5 min before being heated for 15 min at 70°C in a 500-milliliter Erlenmeyer flask with 100 mL sterile distilled water, and filtered. The Ag ions and the GO ions were bio-reduced to NPs by using the filtrate.
Synthesis of amla seed-mediated GO-Ag NPs
The amla seed extract (10 mL) was added to a 1mM aqueous Ag nitrate solution (sample A), and 10 mL of the amla seed extract was added to a 1mM aqueous GO solution (sample B). Both samples were incubated in the dark for 24 h, after which ultraviolet-visible (UV-VIS) spectrophotometry was used to determine the maximum absorbance. To produce GO-Ag nanocomposite samples, an equal proportion of both samples (sample A and sample B) were combined together (sample C). All samples were then heat-dried to obtain the synthesized AgNPs, GONPs and a GO-Ag nanocomposite.
Preparation of the nanocomposite mouthwash
The main component were GO-Ag NPs, which were solubilized by using ethanol as a solvent (0.2%). The clove oil served as the flavoring agent, while sodium benzoate acted as a preservative. Other additives, including sucrose, sodium dodecyl phosphate and distilled water, were also added.
Observational parameters
At baseline (day 0), oral prophylaxis was performed for all patients, and they were provided with the mouthwashes of their respective groups and received similar oral hygiene instructions. The patients were recalled 15 days after baseline. There were no reported side effects from the use of either the test or control solutions. At the baseline and at the revisit (after 15 days), the Silness–Loe PI and Loe–Silness GI were recorded. Gingival crevicular fluid (GCF) and subgingival plaque samples were collected at baseline and at the 15-day follow-up to assess the C-reactive protein (CRP) levels and colony-forming units (CFUs), respectively.
Collection and processing
of gingival crevicular fluid
The crevicular site was dried and isolated with the use of a cotton roll. A standardized volume of GCF was collected by inserting a white color-coded calibrated 1–5-microliter microcapillary pipette from the test site, placing the tip of the pipette extra crevicular. The GCF was immediately placed into plastic vials containing phosphate-buffered saline (PBS) and frozen at 70°C. The CRP levels in the GCF samples were assessed with a sandwich enzyme-linked immunosorbent assay (ELISA) kit (Calbiotech, Inc., El Cajon, USA), following the manufacturer’s instructions.
Collection and processing
of subgingival plaque
At each visit, subgingival plaque samples were taken for microbiological investigation. The sample was transferred to a 5-milliliter Eppendorf tube containing a thioglycolate transport medium. The samples were inoculated into a brain heart infusion (BHI) medium, using the streak culture technique. The plates were incubated at 37°C in an anaerobic jar with a gas-pak sachet. The CFUs were counted after 48 h.
Statistical analysis
The IBM SPSS Statistics for Windows software, v. 23.0 (IBM Corp., Armonk, USA), was used to perform statistical analysis. Intergroup analysis of the mean PI and GI scores, and CRP levels used the independent t test, whereas intragroup analysis at different time intervals employed Student’s paired t test. With regard to CFUs, the Mann–Whitney U test was performed for intergroup analysis, and the Wilcoxon signed-rank test was carried out for intragroup analysis. The level of significance was set at p < 0.05.
Results
Table 1 shows the results of Student’s paired t test and the independent t test for intragroup intergroup comparisons, respectively. There were statistically significant differences in the mean PI and GI scores, and CRP levels in the post-intervention period as compared to baseline in both groups (p < 0.05). Hence, it is evident that the nanocomposite mouthwash and the CHX mouthwash were equally potent in reducing the PI and GI scores, and the CRP levels. Intergroup comparisons showed no statistically significant differences between group A and group B at baseline for PI, GI and the CRP levels. However, after the intervention period of 15 days, there were statistically significant differences between the 2 study groups in terms of mean PI and GI scores, and CRP levels (p < 0.05).
Table 2 shows the mean counts of CFUs at baseline and at 15 days of intervention. The Wilcoxon signed-rank test results showed statistically significant differences in the mean counts of CFUs in the post-intervention period as compared to baseline in both groups (p = 0.001). Hence, we can infer that both the nanocomposite mouthwash and the CHX mouthwash showed similar effects of significantly reducing the mean CFU counts. Table 3 outlines the results of the Mann–Whitney U test, which revealed no statistically significant difference between group A and group B at baseline. However, after 15 days of intervention, the difference between the 2 study groups in the mean count of CFUs showed statistical significance (p = 0.001).
Discussion
The present study compared the antibacterial and anti-inflammatory efficacy of the amla seed-mediated GO-Ag nanocomposite mouthwash and the CHX mouthwash in plaque-induced gingivitis patients. Nanoparticles kill bacteria by creating reactive oxygen species (ROS), such as hydrogen peroxide, superoxide radicals and singlet oxygen. Reactive oxygen species are the products of oxygen metabolism in physiological settings, and play an important role in cell signaling and cellular homeostasis. When metal oxide NPs are in an aqueous medium, they gradually release metal ions. These metal ions can permeate cell membranes, and interact with nucleic acids and protein functional groups. Such interactions have a variety of consequences, including abnormal enzyme activity, cell structure changes, the modification of physiological processes, and microorganism inhibition.20 Chlorhexidine gluconate is considered a standard agent for chemical plaque control. However, its long-term use can have several undesirable side effects. Therefore, the authors of the present study explored the use of a nanocomposite mouthwash as an alternative to a CHX mouthwash.
This study is unique, since there are no previous studies on the antibacterial and anti-inflammatory efficacy of the amla seed-mediated GO-Ag nanocomposite mouthwash. In the present study, it was observed that both the nanocomposite and CHX mouthwashes showed similar effects in terms of reduction of the PI and GI scores. Pradeep et al. carried out a randomized controlled trial to evaluate the efficacy of the triphala (TRP) mouthwash in reducing plaque and gingivitis, and observed that the TRP and CHX mouthwashes were equally effective in improving PI, GI and the simplified oral hygiene index (OHI-S) at all time intervals,21 which is in accordance with the current study. In another study, the turmeric and CHX mouthwashes were used as adjuvants to mechanical plaque control22; the findings were in agreement with the results of the present study. Similar results were reported by Ramamurthy et al.23 Thus, our findings demonstrate that the amla seed-mediated GO-Ag nanocomposite mouthwash possesses anti-plaque and anti-gingivitis properties.
Antibacterial properties were also assessed by evaluating CFUs, with the results showing that the nanocomposite and CHX mouthwash groups had equal significant reductions in the mean CFU count. Komariah et al. assessed the effects of nano chitosan and nano calcium, derived from Xylotrupes gideon, in comparison with the CHX mouthwash.24 Their results were in line with the present study, where both the test and control mouthrinses had comparable potential to reduce total bacterial colonies in the oral cavity. In another study, the authors compared the effects of the GO NP mouthwash and the GO-sodium fluoride (NaF) nanocomposite mouthwash on the Streptococcus mutans (S. mutans) count in the saliva of rats.25 They reported that both mouthwashes efficiently reduced S. mutans in the saliva of rats as compared to the control group.
The mechanism of action behind the antibacterial property of GO involves physical damage to the cell membrane, oxidative stress, and entrapment or wrapping. Furthermore, GO compromises the cell membrane and cell wall integrity. The modified functional groups on GO NPs are thought to play a key role in modulating oxidative stress. Despite this, the precise correlation of functional groups with antibacterial activity has not been well elucidated due to the physical and chemical complexities of GO. Zhao et al. investigated the antibacterial activity of functionalized GO sheets (40 g/mL) on S. mutans.26 The effects of the GO sheet on S. mutans biofilms and S. mutans in a planktonic form were dose-dependent, according to the researchers. Furthermore, GO functional groups were important for the antibacterial action.26 Thus, our findings show that the amla seed-mediated GO-Ag nanocomposite mouthwash possesses an antibacterial property.
C-reactive protein is an acute-phase reactant produced in response to a variety of inflammatory stimuli, such as trauma, infection, heat, and hypoxia. It has a wide range of actions, including pro-inflammatory properties, complement factor activation, the neutralization of the invading pathogens, and tissue regeneration. Periodontitis patients have considerably greater levels of CRP in their serum and GCF than non-periodontitis subjects.27 In addition, there is growing evidence that effective periodontal treatment can reduce the CRP levels. As GO NPs and AgNPs have been proven to have antioxidant and anti-inflammatory properties, they can regulate the CRP levels in GCF.15 Since GCF reflects the ongoing changes in the periodontium, the evaluation of the CRP levels at gingival crevicular sites can be considered a more accurate and less invasive method. In the present study, there were statistically significant differences in the CRP levels in GCF at baseline and at the 15-day follow-up in both study groups. Kumar et al. evaluated the effect of non-surgical periodontal therapy on the CRP levels in GCF, and observed that it was effective in lowering the CRP levels,28 which is in accordance with the present study. Furthermore, the findings of the study supported the hypothesis that the levels of GCF biomarkers are related to the degree of inflammation, collagen degradation and bone turnover.28 In another study, both scaling and root planing (SRP) alone and using a diode laser as an adjunct showed a reduction in the serum CRP levels,29 which is in agreement with the results of the present study. A study by Jayaprakash et al. reported that the CRP levels in GCF gradually increased from a healthy periodontium to gingivitis, and further increased in periodontitis.30 Moreover, it was stated that periodontal therapy reduced the CRP levels.30 Thus, our findings show that the amla seed-mediated GO-Ag nanocomposite mouthwash possesses an anti-inflammatory property.
One of the advantages of the present study is that the baseline parameters were standardized, reducing the impact of gingivitis severity on the effectiveness of the oral rinses. However, this study has some limitations, such as a small sample size and a short follow-up period (15 days). In order to substantiate the study results, a study with a larger sample size and a longer follow-up period should be conducted.
Conclusions
The amla seed-mediated GO-Ag nanocomposite mouthwash efficiently reduced plaque, gingival inflammation and CFUs among patients with plaque-induced gingivitis, but was not equivalent to the CHX mouthwash. Thus, the amla seed-mediated GO-Ag nanocomposite mouthwash can be considered an alternative to the CHX mouthwash.
Ethics approval and consent to participate
The study was approved by the institutional ethics committee at Saveetha Dental College and Hospitals, Chennai, India (IEHC/PERIO-18/2022/35). All participants provided written informed consent.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.