Abstract
Background. Elastomeric chains promote controlled movements and are widely used in orthodontics.
Objectives. The aim of the study was to evaluate the force decay and elongation of orthodontic chains exposed to low-pH saliva (pH = 4) and different beverages common in the diet.
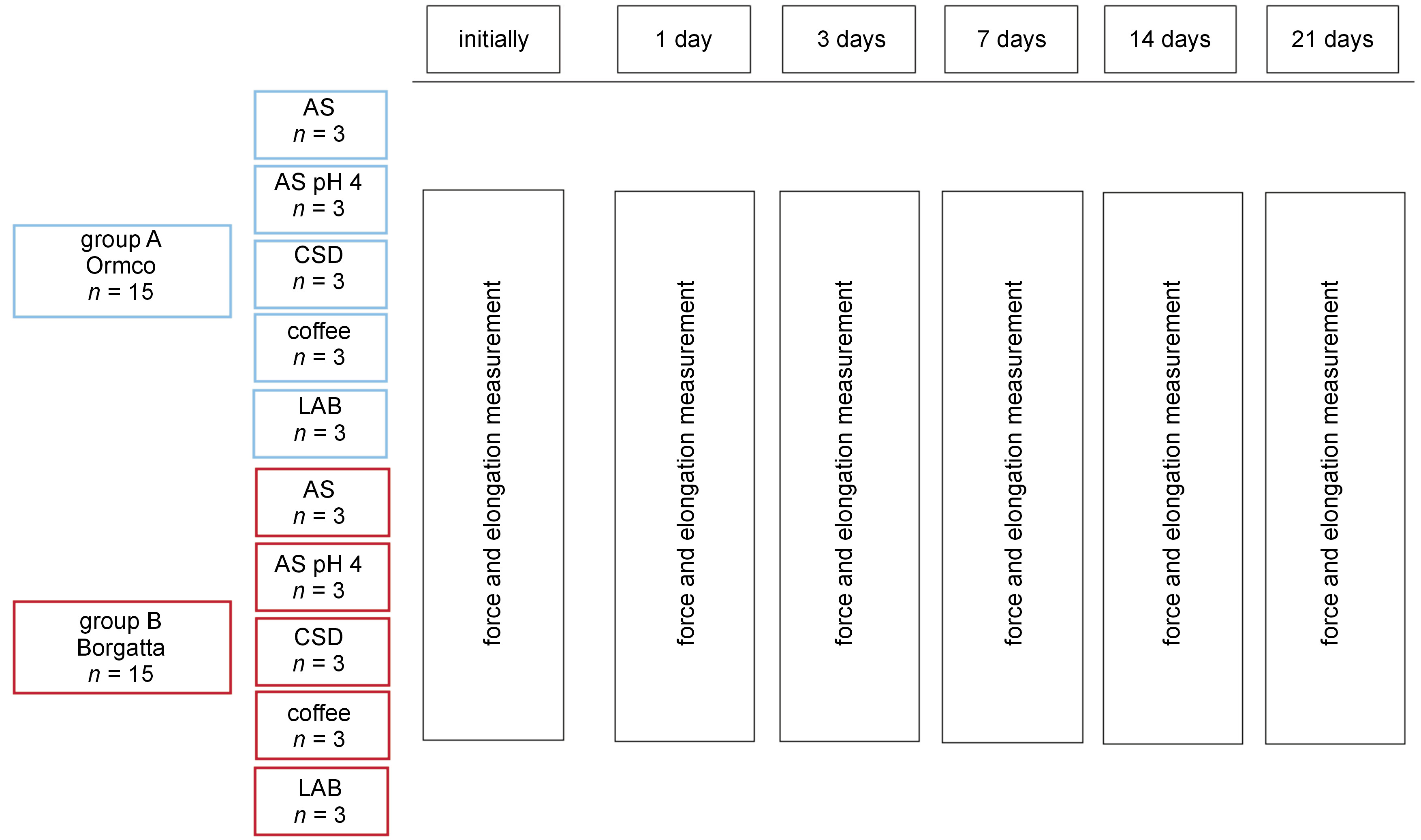
Material and methods. Force decay and elongation were determined in vitro at 6 time intervals over 21 days for 2 commercial elastomeric chains – Ormco (group A) and Borgatta (group B). The samples were immersed in artificial saliva (AS) at pH 4, Coca-Cola®, coffee, or beer for 15 min every day, or in AS (the control group). For the remaining time, the chains were placed into AS at 37°C. In addition, microscopic qualitative changes were recorded by means of scanning electron microscopy (SEM).
Results. The group B chains showed higher force decay and elongation at the end of the follow-up as compared to the group A chains. Exposure to beer had a greater impact on the force decay and elongation of the chains as compared to other liquids, but it was not statistically significant. The group A chains showed a more irregular surface than the group B chains, in particular, those exposed to coffee.
Conclusions. Elastomeric chains suffer force decay and elongation as a function of time, mainly in the first 24 h. At the end of the follow-up, the group A chains exhibited less force decay in comparison with the group B chains. The qualitative observations showed that the chains in group A had a more irregular surface than the chains in group B.
Keywords: surface properties, orthodontics, material testing, fixed appliances
Introduction
In orthodontics, dental biomechanics uses force systems to produce controlled dental movements. Elastomeric chains promote these controlled movements, and are widely used for the closure of dental spaces, canine distalization, the correction of dental rotations and midline discrepancies, and the orthodontic traction of impacted teeth; they serve to hold the arches in brackets and act as a substitute for metal ligatures.1 Although the precise composition of elastomeric chains is an industry secret, polyurethanes are known to be their primary component, and orthodontists have used the chains widely since the 1960s.2
Elastomeric chains are easy to handle, low-cost and do not require patient cooperation. However, the main disadvantages of elastomers are force decay and deformation. After a time, the initial magnitude of the force applied by the chain is reduced, and therefore, the movement of the teeth can decrease or stop. On the other hand, the deformation of the material can be elastic or plastic. When the applied force exceeds the elastic limit of the material, the chain shows a permanent alteration, i.e., chain elongation.3
The properties of the chains may be affected by factors such as the low pH of saliva. Beverages and foods can induce important changes in the oral environment in terms of pH, buffer capacity, titratable acidity, viscosity, and concentrations of calcium, phosphate and fluoride.4 One study provided participants with a carbonated beverage with a pH of 2.45, and showed that 10 min after consuming the drink, the pH on the surfaces of the teeth dropped to 4.5 Likewise, the present study used artificial saliva at pH 4 to mimic the conditions in the mouth after the consumption of an acidic beverage.
Many common drinks have characteristics that can affect elastomeric chains and the oral environment, such as the liquid temperature, a low pH, a high sugar content, artificial coloring, and alcohol content. Coca-Cola® was chosen, as it is a very popular carbonated soft drink (CSD) globally, and has a low pH and a large amount of sugar.6 Coffee is a very popular drink with a low pH that is usually consumed at about 80°C, and can be used to observe the effects of heat and acidity on elastomeric chains. Alcohol can also affect the chains,7 and beer was chosen as a drink with a low alcohol concentration for this study. The present in vitro study aimed to evaluate the force decay and elongation of 2 orthodontic chains exposed to different immersion media.
Material and methods
Groups
Group A (n = 15) used a thermoset closed chain – Generation II Power Chain (Part No./Ref 639-0002; Ormco, Brea, USA).
Group B (n = 15) used a Dentsply Raintree closed chain (SKU:300-044; Borgatta, Ixtapaluca, Mexico).
A diagram of the groups is shown in Figure 1.
Exposure
The control chains were immersed in artificial saliva (AS) at 37°C, without exposure to other solutions. On the other hand, the experimental groups were immersed in AS at pH 4.0 (37°C) to simulate changes in saliva acidity following the consumption of beverages. The experimental groups also included chains immersed in common beverages to simulate daily consumption, including a CSD (Coca-Cola at a refrigeration temperature of about 4°C), coffee (freshly prepared using ground coffee beans in a conventional coffee maker, at a temperature of about 80°C) and a low-alcohol beverage (LAB) (beer at a refrigeration temperature of about 4°C), for 15 min every day, as shown in Figure 1.
Procedure
Roth 0.22-inch slot premolar brackets (REM Orthodontics, Veracruz, Mexico) were cemented on acrylic plates with dimensions of 25 mm, 10 mm and 3 mm in length, width and thickness, respectively, using a previously worn surface. Following the manufacturer’s instructions for each material used, a layer of silane and a layer of Tetric® N-Bond (Ivoclar Vivadent, Schaan, Liechtenstein) were applied, with the latter one light-cured; finally, a portion of Tetric EvoFlow fluid resin (Ivoclar Vivadent) was applied and light-cured. The brackets were placed 10 mm from center to center to emulate canine retraction, with sliding mechanics used to close premolar extraction sites. After evaluating the integrity of cementation, each chain was placed on the bracket and dragged to another bracket on the tension plate. The chains consisted of 3 loops (unit modules) – 2 attached to the brackets and 1 free loop in the middle. The chains were immersed in the different liquid environments for 15 min every day, for 21 days. For the rest of the day, the chains were placed into 10 mL of AS (Viarden, Mexico City, Mexico) in 6-well microplates (Costar® Cell Culture Plate Non-Pyrogenic, model 3506; Corning Life Sciences, Kennebunk, USA) and stored in an incubator at 37°C. The control group chains were also immersed in AS at 37°C the entire time. The AS was replaced every 3 days in all groups to compensate for the liquid evaporation caused by the incubation temperature. After each exposure, the samples were rinsed with 15 mL of sterile saline solution.
The force was measured in grams [g], using a Dontrix tensiometer force gauge (Morelli Ortodontia, Sao Paulo, Brazil). The length measurements of the orthodontic chains were performed with high-precision digital Vernier calipers (Morelli Ortodontia) to determine elongation. The measurements were carried out at the following time intervals: baseline (before placing on the tension plate), and 1 day, 3 days, 7 days, 14 days, and 21 days after the first immersion, as shown in Figure 1.
To maintain blinding, one operator placed the chains and made the measurements, while another analyzed the data without knowing the nomenclature of each group.
Statistical analysis
Descriptive data were expressed as mean ± standard deviation (M ±SD). The Shapiro–Wilk normality test was performed for all data, and the statistical analysis used the Mann–Whitney U test. The IBM SPSS Statistics for Windows software, v. 25.0 (IBM Corp., Armonk, USA), was used for the analysis. Differences were statistically significant at p ≤ 0.05.
Results
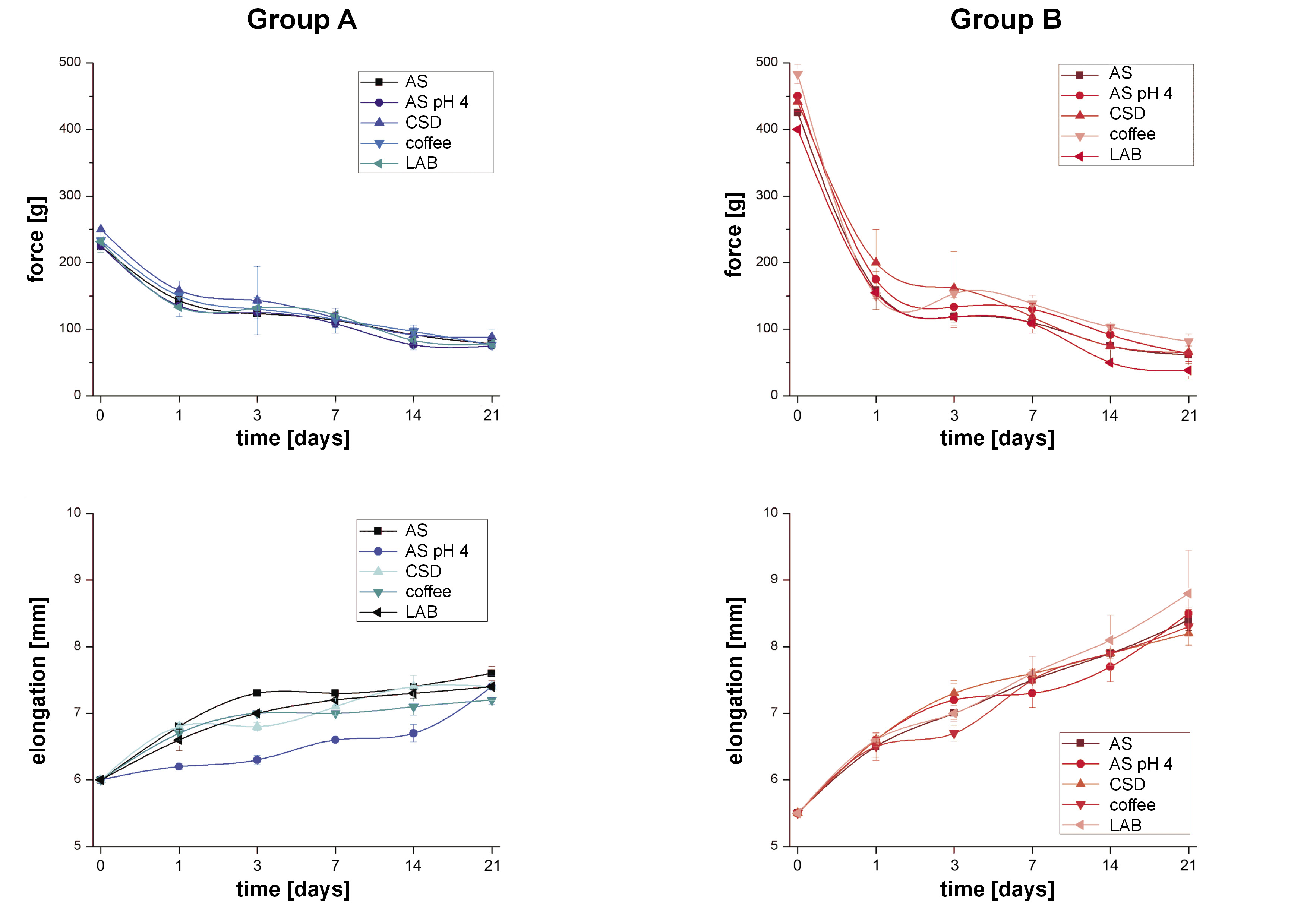
The normality test showed that the force decay and chain elongation data did not follow a normal distribution. The force decay and elongation results are shown in Table 1 and Table 2, respectively, and in Figure 2.
The group A chains immersed in AS at pH 4.0 (z = −1.993; 95% confidence interval (CI): −11.3–17.9; p = 0.561), CSD (z = −1.107; 95% CI: −32.2–12.2; p = 0.268), coffee (z = –0.449; 95% CI: 11.4–14.8; p = 0.653), and LAB (z = –0.449; 95% CI: 11.4–14.8; p = 0.653) exhibited comparable values of force at the end of the follow-up, and there were no significant differences as compared to the control (Table 1).
Group B showed the dispersion of the data with regard to the initial force of the chains. In addition, in the intragroup comparisons, the chains lost a large percentage of strength at the end of the follow-up in relation to baseline in all liquid environments. The group B immersed in AS at pH 4.0 (z = 0.346; 95% CI:= −29.0–25.7; p = 0.692), CSD (z = −0.443; 95% CI: −25.0–18.4; p = 0.658) and coffee (z = –1.623; 95% CI: −47.4–7.4; p = 0.105) exhibited similar force values to the control at the end of the follow-up. At the same time interval, the LAB group had the lowest force value (z = −23.300; 95% CI: 5.2–51.9; p = 0.050) in comparison with the control, which has clinical relevance, since the exerted force could be insufficient (Table 1).
The group B chains showed higher force decay and elongation at the end of follow-up as compared to the group A chains. At the initial measurement, the chains in group A had a greater size than the group B chains. At the end of the follow-up, no significant elongation differences were observed in the group A chains exposed to AS at pH 4.0 (z = –1.528; 95% CI: = –0.1–0.5; p = 0.127) and CSD (z = –1.091; 95% CI: –0.2–0.5; p = 0.275) as compared to the control. The chains exposed to coffee (z = –1.091; 95% CI: 0.10–0.6; p = 0.016) and LAB (z = –1.993; 95% CI: 0.0–0.4; p = 0.046) showed less elongation. From group B, the chains immersed in LAB presented higher elongation (z = –0.886; 95% CI: –1.5–0.7; p = 0.376), but the difference was not statistically significant (Table 2).
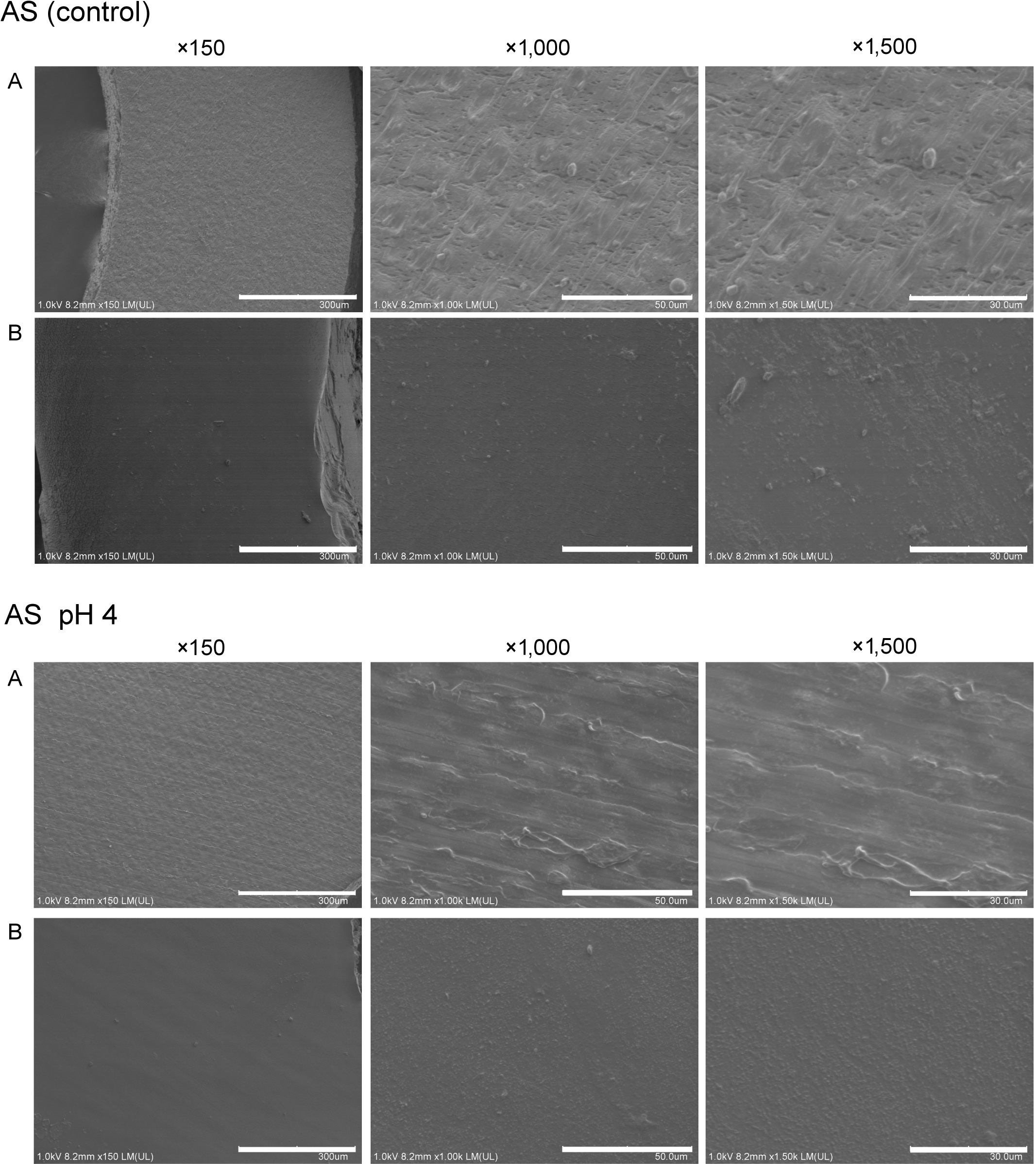
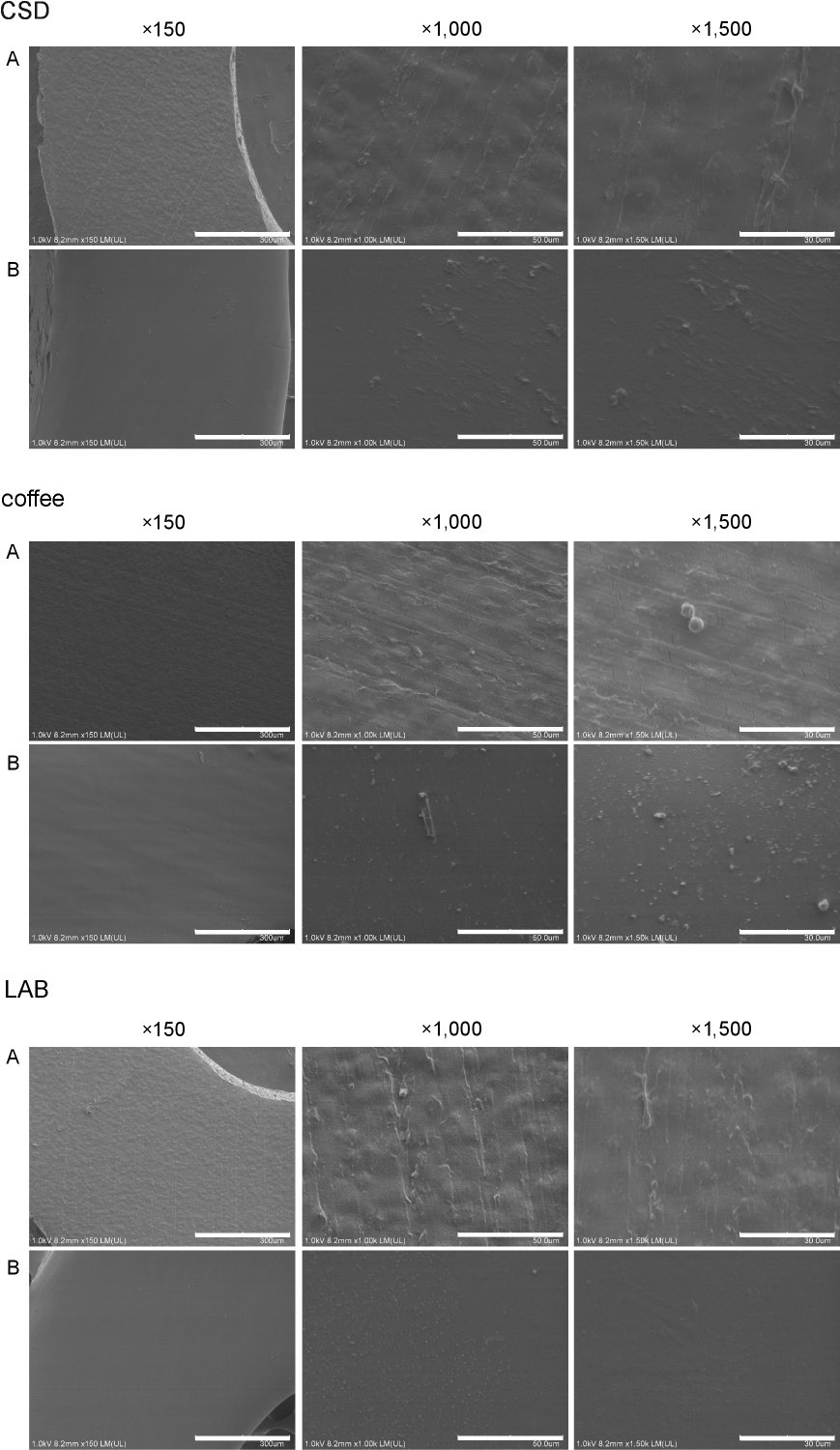
The group A chains immersed in AS had a more irregular surface than the group B chains, with micrographs (×1,500 magnification) showing superficial cracks on the chains. The chains in groups A and B exposed to AS at pH 4.0, CSD and LAB were similar to those in the control group. In contrast, the group A chains exposed to coffee had more visible cracks than those of the control (Figure 3 and Figure 4).
Discussion
In general, orthodontic elastomeric chains are made of polyurethanes, which are polymers synthesized from a polyaddition reaction between a diisocyanate or a polymeric isocyanate with a polyol in the presence of catalysts and additives. An unlimited number of these compounds can be found, with the only condition being the presence of a urethane group (-NH-CO-O-) in the polymer chain. The urethane group is obtained by the reaction between isocyanate and hydroxyl groups, although amines can be used in special cases. For polyurethane elastomers, the following can be obtained by reacting: a diisocyanate (aromatic or aliphatic; RN=C=O), a long-chain diol (R-(OH)2), and a chain-extending low-molecular-weight diol or a diamine (R-(NH)2). These polymers exhibit elastomeric behavior when possessing highly flexible chains, i.e., a low degree of intermolecular interaction and the presence of cross-linked links, which prevent the sliding of the polymeric chains with others. The nature of the bonds can be physical or chemical; the difference between them is the melting or dissolution point of the resulting polymer. Chemical bonds produce an irreversible network that is difficult to destroy by using heat treatment, while physical bonds allow the melting or dissolution of the material.8 Also, the mechanical properties of polyurethane elastomers can be modified by terminal urethane bonds in the copolymer. These elastomers are capable of forming hydrogen bonds with the functional groups of the neighboring molecules, creating a physical grid. Due to this, environmental factors (high temperatures, oxygen concentration, changes in pH, and water absorption, among others) affect the physical properties of elastomeric chains.9, 10
In the present study, important differences were found in the initial force exerted by the chains at the baseline. This finding has been previously reported and attributed to dimensional variations of the elastomeric chain, even within the same brand.11 Regarding their appearance, elastomeric chains can be transparent or colored, with the former used in the current study. It has been postulated that the pigments used in colored chains could contaminate the polymer and cause a decrease in the exerted force.11 It seems that certain colors from a particular manufacturer showed a decrease in the force applied to the tooth; however, the magnitude of such a decrease does not appear to be clinically relevant.12 The forces exerted by these materials are unstable and change over time depending on several factors, including the chain configuration (open or closed) and if the chain is pre-stretched, though this varies according to the speed and amount of stretch. Factors in the oral environment include saliva properties, such as changes in pH, and exposure to light, air, water, oxidants, food, and the rinses used in hygiene,13 as well the application of forces during chewing and brushing the teeth, and the active substances of dentifrices. The effects of the bleaching agents of dentifrices on force decay in elastomeric chains have been reported, with the Sensodyne and Crest whitening pastes showing a greater loss of strength at 28 days than a standard dentifrice,14 but the effects of other pastes containing arginine,15 used for the prevention of caries or tooth sensitivity, on elastomeric chains have not been investigated.
In the present study, the chains were not pre-stretched and were placed using brackets to emulate the clinical conditions. In the same sense, the purpose of exposure to different liquid environments was to simulate a moderate daily consumption of beverages or a low-pH (pH 4.0) salivary environment. At 21 days, the resulting percentage of force exerted by the group A chains immersed in AS was 34%, whereas for the group B chains, it was 14% under the same conditions. In a study performed by Kassir et al., the authors reported 79.8% of force remaining in the chains of the same brand as used in group A; however, they used pins instead of brackets to place the chains.16 As far as we know, there is no study on group B chains.
The results of the present study align with those of Teixeira et al., who demonstrated that strength reduction was more critical in the first 24 h.17 Furthermore, no statistical difference was found after the exposure of the tested elastomeric chains to different acidic environments.17
The CSD showed less force decay for both groups (A and B) as compared to the control at 21 days, which is in accordance with the results reported by Kumar et al., who exposed the chains to a CSD for 1 min twice a day for 28 days.18 Carbonated soft drinks contain carbonated water, water that contains carbonic acid (H2CO3), which is unstable and readily decomposes into water and carbon dioxide (CO2). Carbonated water is responsible for the effervescence of these beverages. Although the release of oxygen could theoretically affect the elastomer bonds, the deterioration of the chains exposed to the CSD was not observed.
Regarding force decay due to the manufacturing process, thermoplastic and thermoset chains have been studied. Thermoplastics are made of a moldable polymer at high temperatures, whereas thermoset chains are irreversibly cured during the fabrication process. The group A chains were thermoset, and their mechanical behavior was more stable. However, the manufacturer of the group B chains did not provide information with regard to this. An in vitro study found that thermoplastic chains showed 20% more force decay when compared to the corresponding thermoset chains of the same brand.19 In vivo, no differences were found between thermoplastic and thermoset chains.20
The current study found that coffee exposure did not result in increased force decay as compared to the control. Braga et al. investigated the effects of hot beverages (hot water, green tea and coffee) on the force decay of chains, and their results showed no statistical difference between the control and coffee groups at 21 days, in contrast with the other hot beverages.21 On the other hand, Aldrees et al. studied the effects of coffee and found yellow discoloration of chains.22
The group B chains exposed to beer showed a higher decrease in force in comparison with the other fluids and the control. Group A did not show a difference in comparison with the control. Larrabee et al. tested the effects of alcohol exposure at concentrations of 14% and 26.9% on elastomeric chains, and their results showed that alcohol significantly decreased the force exerted by chains in a concentration-dependent manner.23 The clinical relevance of the consumption of LABs on the force of elastomeric chains remains unclear.
The force required for orthodontic movement varies according to the type of movement. Low forces have been considered as those <100 cN and high forces as those >150 cN,24 although there is still some controversy about the optimal forces for each movement. In the case of canine retraction, 150 g of force is usually used.25 However, other researchers have applied forces of 100 and 150 cN, and as a result, no significant differences in external apical root resorption and the movement rate were found.26 In the present study, the magnitude of the force exerted by the chains at the end of the follow-up appears to be insufficient for canine retraction regardless of the type of liquid to which the chains were exposed.
A meta-analysis by Andhare et al. determined the proportion of difference in the force decay rate between in vitro and in vivo studies, and reported similar results from both types of studies, implying that in vitro research can replicate clinical conditions.10
Similar physical behavior has been observed in intermaxillary elastics. Large discrepancies have been shown in the physical and mechanical properties of elastics made by the same manufacturer regardless of the type of elastic.27 Several in vitro studies have reported a greater strength loss in latex and non-latex intermaxillary elastics after placing them in the oral cavity.28 Latex elastics presented superior strength than the non-latex ones.29 Leão-Filho et al. evaluated the in vitro effects of frequently ingested beverages (Coca-Cola, coffee, orange juice, beer, red wine, and AS as the control) on the force degradation in intermaxillary elastics (1/4-inch).30 As a result, they found no statistically significant differences between the beverages and concluded that the chemical nature of the beverages was not able to influence the degree of force degradation.30 Likewise, in another study performed by Macedo et al., the authors did not find differences between the latex and non-latex elastics exposed to saliva and chlorhexidine solutions.28
Although the initial force that the chains exert on the teeth is sufficient, the changes occurring after 21 days may render them insufficient. Hence, the clinician needs to educate the patient on scheduling regular appointments. In addition, various strategies have been investigated as future perspectives for improving the efficiency of elastomeric chains. Nanotechnology has been used as a novel approach in dentistry to improve mechanical and biological properties.31, 32, 33 In that sense, nanoparticles have also been developed for orthodontic materials34, 35 to avoid the reduction of elasticity, diminish surface alterations that occur due to the patient’s diet36 and provide antimicrobial activity. Furthermore, another approach consists in incorporating S-Nitroso-N-acetylpenicillamine, a synthetic nitric oxide, into the orthodontic elastomeric chain to generate an antibacterial polymer, which shows good short-term results.37
The present in vitro study did not fully mimic the oral conditions of the patient. The research was not intended to replicate the consumption of each of the most common drinks, since there are too many variables that intervene in the diet of each individual. However, it did compare the elastomeric chains subjected to different beverages to discover which of them produces a greater change in force decay and greater deformation.
Conclusions
Elastomeric chains suffer force decay and elongation as a function of time, mainly in the first 24 h. At the end of the follow-up, the group A chains exhibited less force decay than the group B chains. Furthermore, the qualitative observations showed that the chains in group A had a more irregular surface than the chains in group B.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.