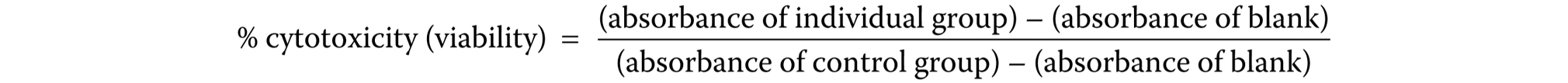Abstract
Background. The information regarding the cytotoxicity of ceramic and resin-matrix ceramic materials subjected to over-the-counter bleaching agents is limited in the literature.
Objectives. The aim of the present study was to investigate the cytotoxic effects of lithium disilicate ceramic (LDC), resin nano-ceramic (RNC) and nano-hybrid composite (NHC) computer-aided design/computer-aided manufacturing (CAD-CAM) block materials subjected to a home bleaching agent and artificial saliva.
Material and methods. A total of 432 specimens were prepared from 3 different CAD-CAM materials. Each material group was divided into 4 groups according to the storage medium (phosphate-buffered saline (PBS) or artificial saliva), and whether the specimens were subjected to a bleaching agent or not. For the bleached groups, hydrogen peroxide (10%) was applied to the specimens for 30 min/day for 15 days, and the specimens were immersed in PBS or saliva after bleaching. The viability of epithelial cells was detected using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay at the end of the 5th, 10th and 15th day of the study. The data was statistically analyzed.
Results. Regardless of the storage medium and the time period, all restorative materials decreased the viability of cells. The highest cytotoxicity levels were determined on the 15th day of the study. The application of a bleaching agent increased the cytotoxicity of the LDC specimens stored in artificial saliva. The RNC material stored in PBS demonstrated significantly higher cell viability than the LDC and NHC groups. The LDC and RNC specimens stored in artificial saliva did not show any significant difference in cytotoxicity. When the materials were subjected to bleaching, NHC demonstrated the highest cytotoxicity during all periods. No significant difference was found between the LDC and RNC specimens subjected to both artificial saliva and bleaching in terms of cytotoxicity.
Conclusions. The type of restorative material, the immersion medium, the application of a bleaching agent, and the application period affected the cytotoxicity of the materials. Over-the-counter home bleaching agents may induce cellular cytotoxicity due to the existing restorations, and patients should be informed about this potential biological response.
Keywords: cytotoxicity, lithium disilicate, home bleaching, resin nano-ceramic, nano-hybrid composite
Introduction
In the last decade, the dental industry has focused on the development of new materials with improved optical properties due to the increased demands and expectations of patients regarding esthetic appearance. Computer-aided design/computer-aided manufacturing (CAD-CAM) systems provide the standardized and controlled milling of different types of restorative materials. Ceramics, resin composites, resin-matrix ceramics, and polymethyl methacrylate (PMMA) are indirect restorative materials available as pre-processed blocks. Precisely fitting restorations can be fabricated via the CAD-CAM technology, using these homogenous and defect-free blocks.1 Among the aforementioned materials, resin-matrix ceramics are relatively new; they have become popular in the last few years. Ceramics show improved optical characteristics, higher biocompatibility, stain resistance, and durability in comparison with resin composites. However, resin composites provide lower abrasion on the antagonist enamel or restorative material, a lower modulus of elasticity, and better polish and repair properties than ceramics. Moreover, the lower brittleness and chipping fracture incidence of resin composites are advantageous when the material is subjected to milling. Therefore, resin-matrix ceramics, which are aimed to combine the beneficial properties of ceramics and composites, are preferred for chairside CAD-CAM restorations.2 Lava™ Ultimate is the first material introduced as a resin nano-ceramic (RNC) containing silica (Si) and zirconia (Zr) nanoparticles (80 wt%) embedded in a highly cross-linked polymer matrix (20 wt%) composed of bisphenol A glycidyl methacrylate (BisGMA), urethane dimethacrylate (UDMA), ethoxylated bisphenol A dimethacrylate (BisEMA), and triethylene glycol dimethacrylate (TEGDMA).3
In previous studies, the mechanical and optical behavior of resin-matrix ceramics was investigated for a better understanding of their clinical performance, and these materials were compared to ceramics and conventional resin composites.3, 4, 5, 6, 7 The mechanical strength of resin-matrix ceramic blocks was reported to be superior to conventional composites,8 while the flexural properties were found to be comparable to glass ceramic, but inferior to lithium disilicate ceramic (LDC) blocks.9 Therefore, the properties of resin-matrix materials were considered to be between those of ceramics and conventional resin composites.10
At-home and in-office tooth bleaching are widely used procedures to improve the esthetic appearance by eliminating the discoloration of teeth.11 Higher concentrations of hydrogen peroxide are used during in-office bleaching under the observation of the clinician, while at-home bleaching is performed by the patient with lower concentrations of carbamide peroxide or hydrogen peroxide.12, 13 During these procedures, not only the
surfaces of the teeth, but also the existing restorations are subjected to bleaching agents.14, 15, 16 In this sense, the effects of these bleaching agents on the optical properties and surface characteristics of ceramics and resin composites were investigated in several studies,15, 16, 17, 18, 19 and the influence of the bleaching procedures was concluded to be material-dependent.20 One of the previous studies reported that the bleaching procedures with high-concentration agents increased the surface roughness of RNC materials.21 Such surface alterations may result from water absorption or the loss of inorganic filler particles, caused by the diffusion of the free radicals released from peroxides into the resin matrix.22 It has been documented that bleaching increases the release of monomers from resin composites,23, 24 which may also change the surface topography. Since these monomers are reported to be cytotoxic,25 and as they can be released into the saliva after bleaching and contact oral tissues, the behavior of restorative materials that are subjected to bleaching should be known. Therefore,
the present study aimed to investigate the cytotoxicity effects of a home bleaching agent (10% hydrogen peroxide) applied to LDC, RNC and nano-hybrid composite (NHC) CAD-CAM blocks in contact with epithelial cells. The null hypotheses of the study were as follows:
(1) storage in artificial saliva and (2) the application of a bleaching agent would not affect the viability of the cells in contact with the restorative materials; (3) there would be no significant differences in the cytotoxicity levels of the restorative materials; (4) the duration of the storage of the restorative materials in artificial saliva and (5) the duration of bleaching agent application would not affect the cytotoxicity of the tested materials.
Material and methods
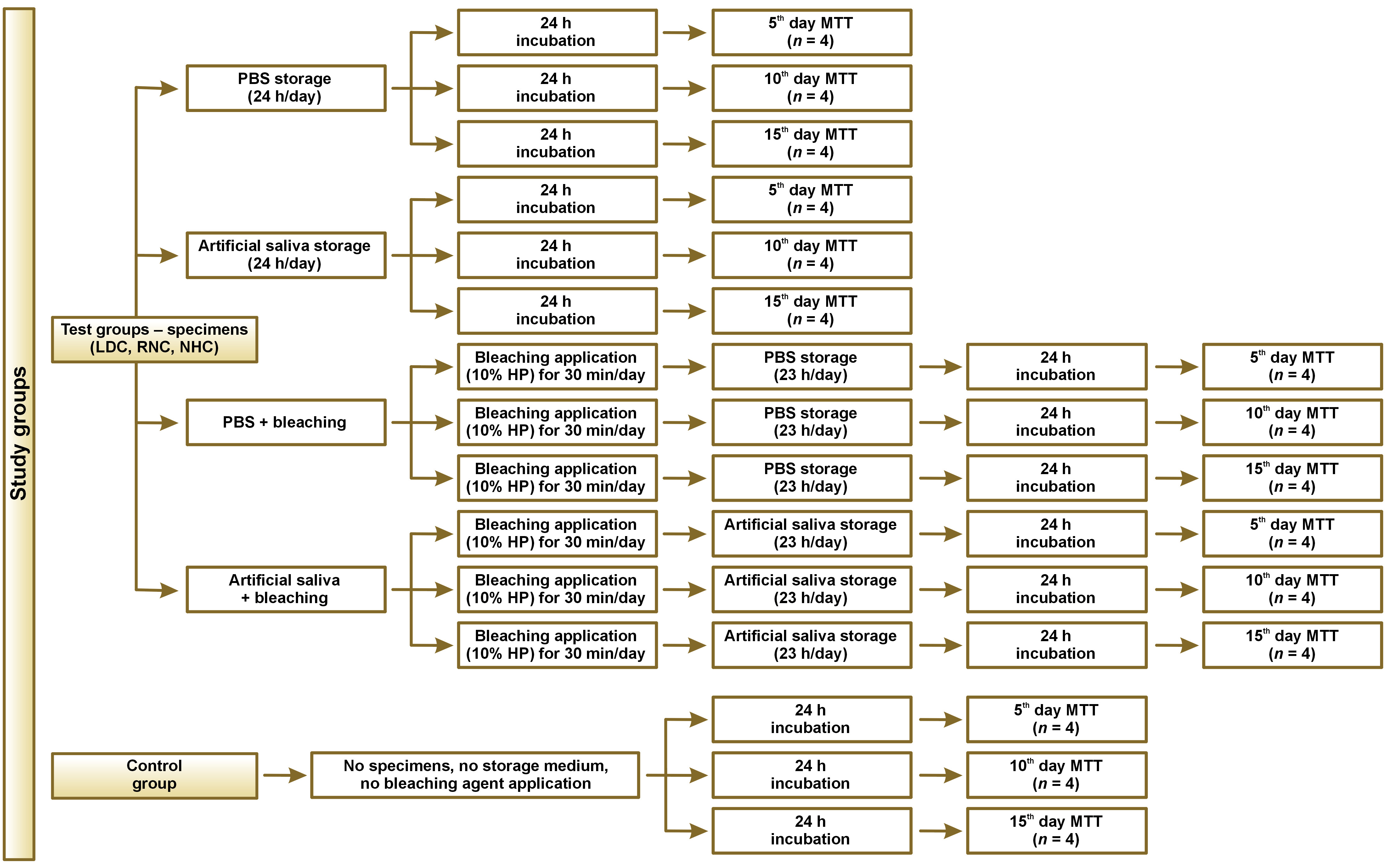
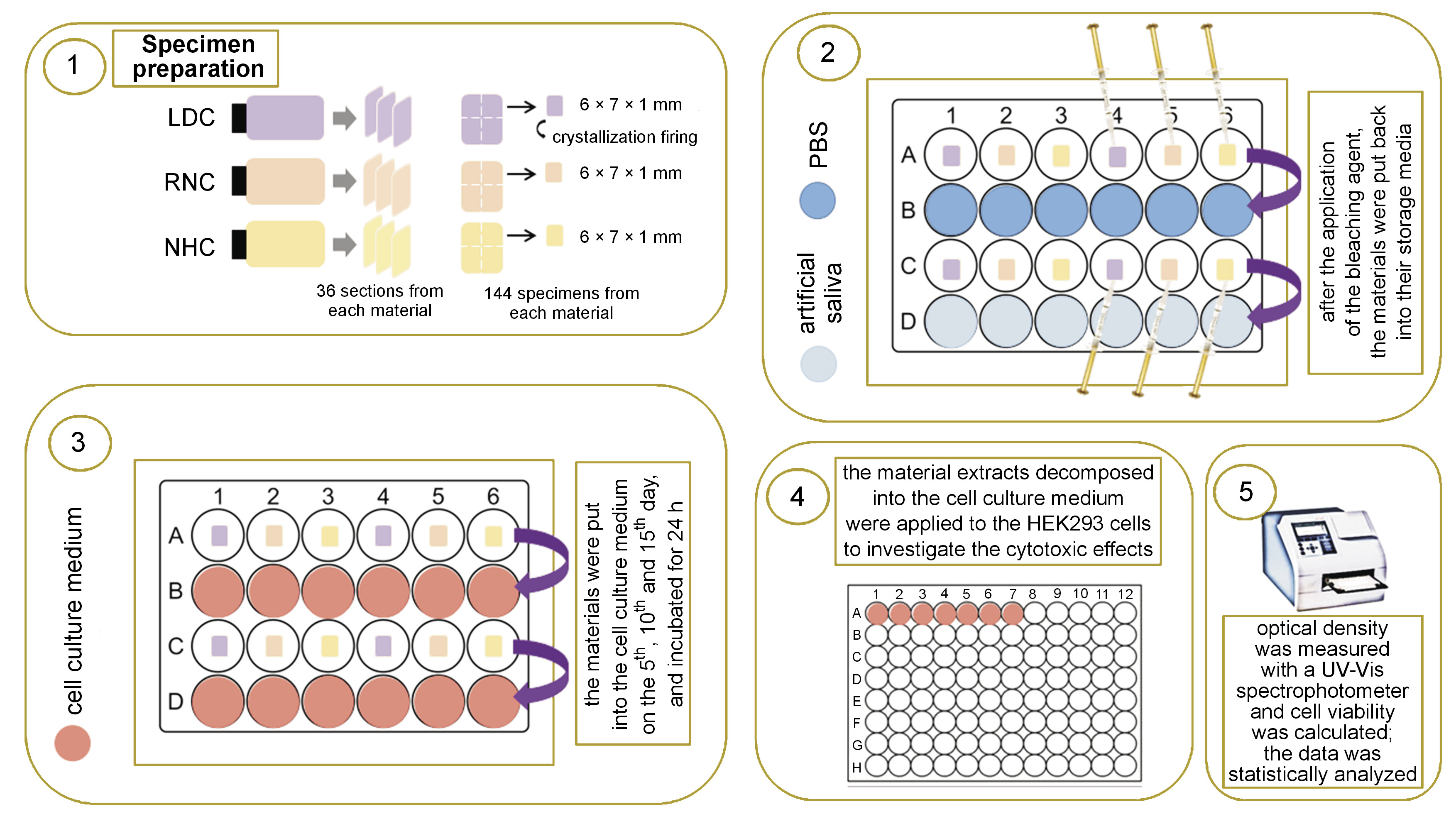
The study design and test procedures are presented as a flowchart in Figure 1 and are schematically illustrated in Figure 2.
Specimen preparation
Specimens of LDC, RNC and NHC of the same shade (A2) were evaluated. The composition and manufacturers of the CAD-CAM restorative materials are presented in Table 1. Thirty-six specimens of a rectangular shape and a thickness of 1.2 mm were obtained from each material (a total number of 108 specimens), using CAD-CAM blocks and a low-speed diamond saw (IsoMet™; Buehler, Lake Bluff, USA) with water cooling. By using a diamond disk (Sunshine Diamond; Dr. Hopf GmbH & Co. KG, Langenhagen, Germany), each specimen was sectioned into 4 equal parts with dimensions of 6 mm × 7 mm × 1.2 mm. Thus, a total of 432 specimens were prepared. The LDC specimens underwent crystallization firing (Programat EP5000; Ivoclar Vivadent, Schaan, Liechtenstein) according to the manufacturer’s instructions. All the surfaces of the specimens were ground and polished using under water irrigation with wet silicon carbide papers, following a sequence of 500, 1,200, 2,000, and 4,000 grit to achieve a thickness of 1 mm. The dimensions of the specimens were controlled with a digital caliper (N48AA; Maplin, Rotherham, UK). The specimens were divided into 4 equal groups for each material according to the applied test protocol, as displayed in Table 2. Each group of specimens was further divided into 3 equal subgroups according to the test period (the 5th, 10th and 15th day; n = 4). The specimens in groups 1–6 were not subjected to bleaching, and were stored in either phosphate-buffered saline (PBS) (groups 1–3) or artificial saliva (1.5 mM CaCl2, 0.9 mM KH2PO4, 130 mM KCl, 1 mM NaN3, and 20 mmol/L HEPES) (groups 4–6) during the testing procedures (Table 2).
Application of a bleaching agent
The whole process was carried out in a cell culture cabin (Class II) in a sterile environment and all specimens were autoclaved with a conventional glassware protocol at 121°C for 20 min for sterilization prior to the bleaching procedures. An over-the-counter and prefilled tray-type home bleaching system (Opalescence Go; Ultradent Products Inc., South Jordan, USA) containing 10% hydrogen peroxide was used in this study. A syringe was used to apply an equal amount of the agent on one surface of the specimens and a sterile Heidemann spatula was used to spread the agent uniformly on the surface. According to the protocol recommended by the manufacturer, the application period was 30 min per day. After the bleaching procedure was terminated, the specimens were cleaned and rinsed with PBS. Afterward, the specimens were either put into PBS (groups 7–9) or artificial saliva (groups 10–12) (Table 2). This procedure was repeated for 15 days. At the end of each time period (the 5th, 10th and 15th day), the specimens in their related period subgroups were put into the prepared complete cell culture medium and incubated for another 24 h. Then, the cell culture medium was collected and used for the cytotoxicity study.
There were also control groups consisting of cells that were not in contact with any restorative material, storage medium or bleaching agent; these cells were incubated during the test period. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Acros Organics, Morris Plains, USA) was performed in the control groups at the end of the 5th, 10th and 15th day.
Cultivation of HEK293 cells
Previously cryopreserved HEK293 human embryonic kidney epithelial cells were thawed in a 37°C water bath and transferred into Falcon® tubes for centrifugation at 1,000 rpm for 5 min. After the centrifugation step, the cells were transferred to 75 cm3 flasks with a medium consisting of Dulbecco’s Modified Eagle Medium/F12 (DMEM/F12) (Gibco™, Visp, Switzerland), supplemented with 10% fetal bovine serum (FBS) (Gibco), 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco). The cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and subcultured every 2 days. After reaching 80% confluence, the cells were trypsinized with trypsin-EDTA (Capricorn Scientific, Ebsdorfergrund, Germany) for 10 min at 37°C and 5% CO2 before being used in the cytotoxicity assay. The cells from passage 3 were used in the study.
In vitro cytotoxicity assay
Cytotoxicity was determined by performing the MTT assay to investigate the activity of mitochondrial enzymes in viable cells. Viable cells can successfully cleave MTT and form formazan crystals with the help of a cellular enzyme succinate dehydrogenase (SDH). The formed formazan crystals are later dissolved by dimethyl sulfoxide (DMSO) to terminate the MTT assay. For this purpose, the cells were trypsinized and seeded into 96-well plates at a density of 2 × 105 cells/well, and incubated for 24 h at 37°C and 5% CO2. Then, the culture medium was replaced with the medium subjected to a 24-hour incubation period with specimens that were treated differently (Table 2). The cells were incubated with the replaced culture medium for another 24 h before the termination of the study. After 24 h, the MTT solution (1 mg/mL) was added to the cells and they were incubated for 4 h. The images of the cells were taken at the end of the study by using an inverted microscope (Olympus, Tokyo, Japan). The crystals formed by viable cells were dissolved by the addition of 50 µL of DMSO to each well during the post-incubation period. All the different groups were studied in quadruplicate. An ultraviolet-visible (UV-Vis) spectrophotometer (wavelength λ = 570, Multiskan® Spectrum; Thermo Scientific, Waltham, USA) was used to measure the optical density of the dissolved material. The cellular cytotoxicity rate [%] was determined with the following formula (Equation 1):
Statistical analysis
The data was analyzed with the standard error of the mean (SEM) method, and the statistical significance of differences was examined using the one-way analysis of variance (ANOVA) and Tukey’s post-hoc tests for multiple intragroup comparisons. Comparisons of groups at different time intervals (on the 5th, 10th and 15th day) were performed via conducting the multiple ANOVA with the use of GraphPad Prism, v. 5.0 (https://www.graphpad.com), and IBM SPSS Statistics for Windows, v. 20.0 (IBM Corp., Armonk, USA). The p-values of less than 0.05 were stated as statistically significant.
Results
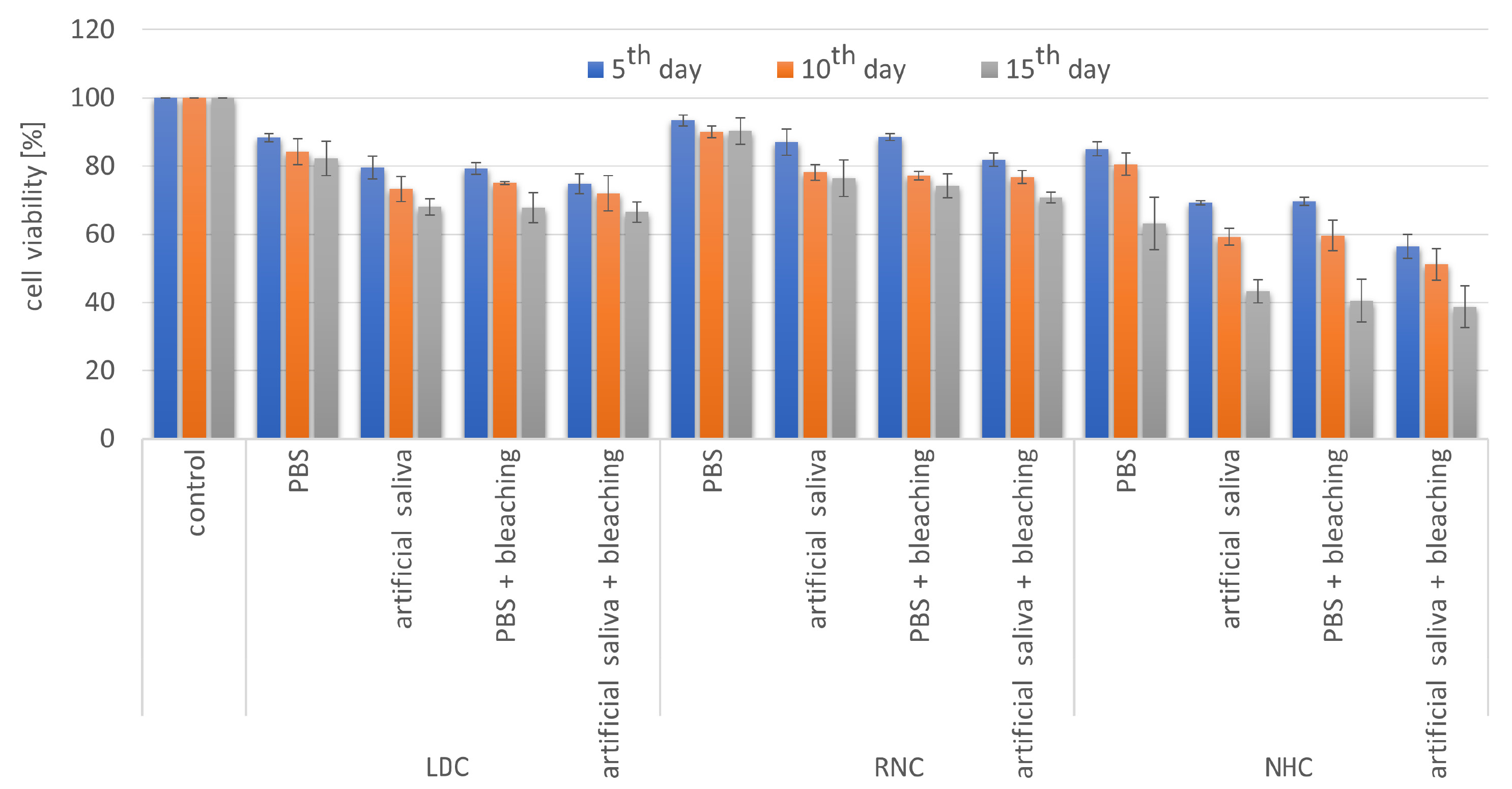
Regardless of the storage medium and the time period, all test groups showed significantly higher cytotoxicity than the control group (p < 0.05) except for RNC immersed in PBS (p > 0.05), which showed the lowest cytotoxicity level among all test groups for all time intervals. The lowest cell viability was detected in the NHC groups subjected to PBS/artificial saliva and the bleaching agent for 15 days (Figure 3).
Within the LDC groups, no significant differences were found among the PBS, artificial saliva and PBS + bleaching groups (p > 0.05), while the artificial saliva + bleaching group demonstrated significantly lower cell viability for all periods (p < 0.05). When the periods were compared, no significant differences were detected between the
5th and 10th day; however, cell viability at the end of the 15th day was significantly lower than that of the other periods (p < 0.05) for all LDC groups except for the PBS group, where the period parameter did not affect cell viability (Table 3).
The RNC specimens stored in PBS showed significantly lower cytotoxicity as compared to the other RNC groups (p < 0.05), which showed similar cell viability for all periods (p > 0.05). When the periods were compared for the RNC material, significant differences were detected between the 5th and 15th day for all test groups except for the PBS group (Table 3).
For the NHC material, the PBS group demonstrated remarkably higher cell viability than the other test groups for all periods (p < 0.05), whereas there were no significant differences among the other groups (p > 0.05). Period comparisons revealed that cell viability at the end of the 5th and 10th day was not statistically different (p > 0.05), while the 15th day data showed significantly lower cell viability for all test groups (p < 0.05) (Table 3).
The restorative materials were compared with regard to each storage medium and the presence or absence of bleaching to investigate the cytotoxicity of materials subjected to the same protocol. The RNC material stored in PBS showed significantly higher cell viability than the LDC and NHC materials for all periods (p < 0.05). No significant differences were found between LDC and RNC stored in artificial saliva (p > 0.05); however, significantly lower cell viability percentages were obtained for the NHC material regardless of the period (p < 0.05). When the materials were subjected to a bleaching agent and stored in PBS, the cytotoxicity induced by NHC was significantly the highest (p < 0.05), while significantly the lowest values were obtained for RNC (p < 0.05). On the other hand, the LDC and RNC specimens bleached and stored in artificial saliva did not show statistically significant differences in terms of cell viability (p > 0.05). However, NHC demonstrated significantly lower cell viability for all periods (p < 0.05) (Table 3).
Discussion
According to the statistical analysis of the data, all the restorative materials subjected to artificial saliva revealed lower cell viability in comparison with the control group, and thus the 1st null hypothesis of the study was rejected. The 2nd null hypothesis of the study was partially accepted, since only the LDC specimens subjected to bleaching and stored in artificial saliva demonstrated significantly lower cell viability than the control group and the specimens from the other LDC groups. The 3rd null hypothesis was rejected, since significant differences in cytotoxicity were detected between the restorative materials, depending on the storage medium and the application of a bleaching agent. Immersing the bleached and non-bleached restorative materials in artificial saliva for different periods of time affected cell viability; therefore, the 4th and 5th null hypotheses were also rejected.
At-home bleaching systems can be categorized as professionally supervised and over-the-counter whitening products.26 The former ones include dentist supervision and provide more controlled application with the use of customized whitening trays.27 Over-the-counter systems do not require dentist supervision and are preferred by patients26 due to a shorter application time.28 The trays used for over-the-counter systems are not customized, and thus the tray cannot fully adapt to the dental arch and provide adequate sealing. This may cause the overflow of the bleaching agent into the oral cavity and its contact with tissues.29 Therefore, the impact of this type of bleaching kits on the biological responses of intraoral tissues should be evaluated.
Besides assessing the mechanical and optical properties of restorative materials, the in vitro determination of their cytotoxicity is a very crucial step in investigating the occurrence of hazards and any cellular problems they may cause.30 The cytotoxicity of these materials might be enhanced when they are subjected to bleaching agents and contact the saliva. The saliva was reported to be responsible for the biodegradation of resin-based materials.31 For this purpose, the present study was conducted to assess the cytotoxicity of different restorative materials subjected to both a 10% hydrogen peroxide bleaching agent and artificial saliva. Cell viability was detected by investigating the mitochondrial activity (the MTT assay) after 5, 10 and 15 days of bleaching agent application. Although the manufacturers of bleaching agents recommend the use of the products for 5–10 days, in previous studies evaluating the clinical outcomes of bleaching, the agent was applied for up to 15 days.29, 32, 33 Therefore, in the present study, 15 days of usage was also simulated, considering the possible over-treatment.
All the evaluated restorative materials were also immersed in PBS to assess and compare the biocompatibility of the materials without bleaching agent application. Besides, PBS was aimed to serve as a control to artificial saliva. The RNC specimens showed significantly the highest cell viability among the tested materials for all periods, followed by LDC and NHC (Table 3, Figure 3). This finding is in accordance with the results of a recent study, which reported that the same RNC material exhibited higher HEK293 epithelial cell viability than LDC at the end of the 7th day.34
Although ceramics are known as inert and biocompatible materials with no cytotoxic effects,35 the suppressed cellular activity caused by LDC was reported in previous studies.36, 37 This cytotoxic response has been related to mass release from the material. The presence of zinc (Zn) in LDC may influence cytotoxicity, since this element is considered a cellular-viability suppressor.38 Previous studies also reported that the cytotoxicity of LDC decreased with time,36, 37, 39 which was possibly due to the fact that the surface of the material adapted to the organic environment at the end of a two-weeks period.36 Distinctively, in the present study, cell viability did not decrease in the LDC group immersed in PBS at the end of the test period, but it was also the case in the RNC group. However, the bleaching procedure caused a significant decrease in cell viability, possibly due to the alteration which occurred on the LDC surface treated with the bleaching agent.
Ceramics may leach and etch simultaneously when exposed to the saliva, and different ions may be released,40 which results in biological responses, depending on the element type. Resin-based materials are also affected by contact with the saliva in terms of monomer release, since the saliva enhances the decomposition of monomers from the surface of the material. The exposure of resin-based materials to artificial saliva for 2 weeks demonstrated a further increase in the release of the decomposed monomers, and thereby increased the cytotoxic effect on epithelial cells.41 Therefore, the restorative materials tested in this study were subjected to artificial saliva for the assessment of the influence of the saliva on cell viability with or without bleaching agent application. For all the evaluated restorative materials, significant differences were found in cell viability between the 5th and 15th day in the artificial saliva-only groups, and these viability values were significantly lower as compared to the control group. These results may be attributed to the release of elements or monomers from the restorative materials, which could be cytotoxic to epithelial cells. The artificial saliva used in the present study included sodium azide (NaN3), which has been shown to reduce cell viability in high concentrations.42 Despite the fact that a non-toxic concentration was used in this study (1 mM), the cytotoxic behavior of artificial saliva may be attributed to the content of this compound.
Although a lot of research has been conducted regarding the cytotoxicity of restorative materials, a limited number of those studies referred to the impact of bleaching agents in this respect. It is recommended to replace the existing restorations or re-polish their surfaces after bleaching to prevent discoloration or plaque accumulation, which may occur due to the surface alterations caused by the bleaching procedure.43 Since over-the-counter bleaching products are not applied under the supervision of the clinician, their effects on the physical, optical and biological properties of restorative materials are of great concern. Therefore, this type of bleaching agents was preferred in the present study.
The specimens exposed to hydrogen peroxide for 30 min were immersed in either PBS or artificial saliva for the rest of the day after removing the bleaching agent from their surfaces. Regardless of the storage medium, all the bleached restorative materials significantly decreased cell viability as compared to the control group. However, when the cytotoxicity of the bleached and non-bleached materials immersed in artificial saliva was evaluated, significantly lower cell viability was detected in the LDC group, which indicates that the cytotoxicity behavior of RNC and NHC did not depend on the application of the bleaching agent. The lowest cell viability observed in the NHC groups can be related to the release of monomers from the material, induced by bleaching agent application and/or immersion in artificial saliva. The NHC material tested in the present study is composed of some monomers, of which BisGMA, UDMA25 and TEGDMA25, 44 have been reported to have cytotoxic effects on certain cell types.25 Volk et al. indicated that TEGDMA in combination with hydrogen peroxide significantly decreased the viability of human oral cells, even in low concentrations.45 Therefore, the significant decrease in the viability of cells exposed to the bleached NHC may be attributed to the cytotoxic effects of the monomers released from the material.
The RNC material exhibited biocompatible behavior, with higher epithelial cell viability values, which is in accordance with recent studies.34, 46 Although RNC includes monomers such as BisGMA, UDMA, BisEMA, and TEGDMA, similar to NHC, the highly cross-linked polymer content of the material is 20%, which is lower than in the case of NHC, and RNC includes Zr particles. Moreover, the fabrication of RNC blocks is carried out under well-controlled temperatures and pressures, which enhances the final polymerization through eliminating shrinkage45 and ensures that the UDMA monomer is bonded to the ceramic network with high strength.47 Thus, although both RNC and NHC have resin content, the materials display different cytotoxicity behavior, which can be explained by differences in the microstructures and manufacturing processes of the materials.
Limitations
In the present study, the surfaces of the specimens were polished to obtain a standardized surface and to eliminate any irregularities. However, invisible porosities or cracks might exist or may have occurred due to bleaching agent application, and these irregularities could cause the storage of hydrogen peroxide, even after the cleaning procedure, which might have affected cell viability. Nevertheless, it should be taken into consideration that such surface irregularities can also be found on the surfaces of restorations.
In the present study, to better simulate the oral conditions, the assays were conducted using extracts from the culture medium and not by direct contact. A colorimetric MTT assay was performed to evaluate the viability of human embryonic kidney epithelial cells. Since the cytotoxicity was evaluated with the use of only the MTT assay, this may be regarded as a limitation, and other cytotoxicity tests involving gingival epithelial cells should be carried out for a better evaluation in future studies.
The determination of the release of monomers or elements, as well as scanning the surfaces of the materials after applying the test protocols can reflect the effect of the bleaching procedures on the materials in a more interpretive way, and can be the subject of further investigations.
Another limitation was the surface finishing of the LDC material, as LDC surfaces are glazed before clinical use; this may have affected the behavior of LDC. However, all the materials were subjected only to polishing in order to supply a standard protocol.
Conclusions
Within the limitations of the study, it can be concluded that over-the-counter home bleaching agents decrease cell viability when in contact with the LDC material. Cell viability was time-dependent, and significantly decreased at the end of the 15th day for all the bleached and non-bleached materials. Storage media and bleaching agents may affect the cytotoxicity behavior of restorative materials. Patients should be informed about these potential biological responses to over-the-counter whitening products and shorter periods of use should be recommended to minimize the cytotoxic effects.
Ethics approval and consent to participate
Not applicable.
Data availability
All data generated and/or analyzed during this study is included in this published article.
Consent for publication
Not applicable.