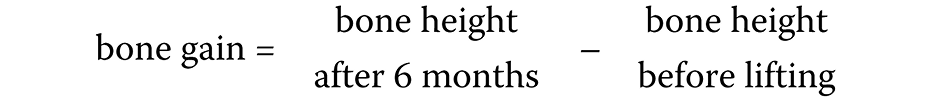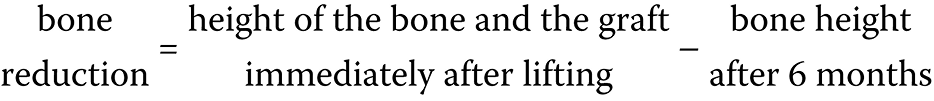Abstract
Background. Maxillary sinus grafting is considered the most common surgical technique to secure a sufficient bone height for placing dental implants. It is carried out either by making a bony window in the lateral wall of the maxillary sinus (the external procedure) or through the alveolar entrance technique by using alveolar osteotomes (the internal procedure), depending on the quality and quantity of the remaining bone.
Objectives. The aim of the present study was to compare radiologically the amount of bone gain (an increase in bone dimensions) and bone reduction (the loss of the graft volume) obtained by using tricalcium phosphate (TCP) and calcium sulfate (CS) grafts mixed with advanced platelet-rich fibrin (A-PRF).
Material and methods. Nine patients (18 maxillary sinuses) participated in this study, all of whom had bilateral edentulism involving the premolar/molar areas and a bone height of 0.5–5 mm between the sinus floor and the alveolar ridge. Two biomaterials were used in the sinus augmentation procedures. Each patient underwent a bilateral maxillary sinus lift with the use of different bone graft materials – with CS mixed with A-PRF used on one side, and TCP mixed with A-PRF on the other side. The grafting site was selected randomly. Afterward, bone gain and bone reduction were evaluated at the grafting site by using cone-beam computed tomography (CBCT).
Results. The mean bone gain on the side treated with TCP mixed with A-PRF was 7.532 ±1.150 mm, and on the side treated with CS mixed with A-PRF side it was 7.961 ±2.781 mm. The comparison of bone gain and bone reduction between the 2 groups showed no statistically significant differences at a 6-month follow-up.
Conclusions. Using CS or TCP mixed with A-PRF was beneficial and safe in the two-stage maxillary sinus lifting procedure. A sufficient amount of bone was obtained for dental implantation.
Keywords: dental implant, sinus lift, bone graft, calcium sulfate, tricalcium phosphate
Introduction
Bone height in the maxillary posterior edentulous area may be insufficient for dental implant placement due to the pneumatization of the maxillary sinus after the extraction of the teeth.1 Maxillary sinus grafting is the most commonly used surgical technique for securing bone height sufficient for dental implantation in such cases.2 The operation is carried out using one of the 2 basic techniques, i.e., making a bony window in the lateral wall of the maxillary sinus (the external sinus lift) or through the alveolar approach with the use of alveolar osteotomes (the internal sinus lift).3
Many types of bone grafts have been used, though autogenous bone has always been considered the gold standard due to superior osteoconduction, osteoinduction and osteogenesis.4 However, there are some disadvantages connected with the process of acquiring it, including the limited amount of bone obtained from the inside of the oral cavity and the need for an additional surgical procedure, which leads to an increase in the surgery time. These factors have led to increasing interest in the search for alternative graft materials.5 Tricalcium phosphate (TCP) is a bone substitute that promotes bone growth4 and is considered one of the preferred grafts for maxillary sinus lifting due to its suitable absorption nature and volume stability.6
Calcium sulfate (CS) occupies a unique position in the field of regenerative materials, as it has a long history of clinical usage as compared to other currently available biomaterials and is widely recognized as a well-tolerated material with applications in bone regeneration. It undergoes virtually complete resorption in vivo, without eliciting a significant inflammatory response,7, 8, 9 which is critical in this procedure, as the positioning of implants seems to be even more delicate. In particular, recent studies have highlighted extremely high levels of peri-implant tissue inflammation as compared to the natural tooth, which promotes long-term bone remodeling and resorption. Using drugs that contain inflammation-moderating components may also enhance the properies of the bone.8
Studies have investigated mixing alloplastic grafts with platelet-rich fibrin (PRF) to reduce the amount of graft and promote osteogenesis in the grafted area. The texture resulting from such mixing facilitates clinical handling, increases the stability of the graft and improves the outcomes.10, 11
This study aimed to evaluate and compare the benefits of using CS and TCP as graft materials for the two-stage maxillary sinus lifting procedure in cases of high bone resorption, using the radiological analysis of bone gain and bone reduction.
Material and methods
Study design
This was a randomized (1:1), split-mouth clinical trial (randomized controlled trial – RCT) (No. Faculty of Dentistry/RCTs-758) comparing the use of CS and TCP in bone grafting for external sinus lifting for dental implantation.
The Consolidated Standards of Reporting Trials (CONSORT) statement was used as a guide for this study.12 The study was conducted in the laboratory of the Maxillofacial Surgery Hospital and the Department of Implantology at the Faculty of Dentistry of Damascus University, Syria (Figure 1).
Informed consent was obtained from the participants, and the ethics board at the Faculty of Dentistry of Damascus University, Syria, approved the study (FMD-185).
Participants
The sample was selected from among the patients who sought implant treatment at the Department of Oral and Maxillofacial Surgery at the Faculty of Dentistry of Damascus University, Syria. Data was collected from February 2018 to January 2021.
Sample size calculation
The sample size was calculated using the G*Power 3.1.3 program (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower), based on a study power of 80% with a significance level at p = 0.05, and the effect size data (0.83) from a study by Călin et al.13 Considering sample dropout led to the addition of 2 maxillary sinuses to each group to give a total sample size of 20 maxillary sinuses. The sample size for each group was 20 maxillary sinuses (10 patients).
Randomization
The maxillary sinuses were randomly allocated using Microsoft Excel 2010 to either the CS group (the intervention group) or the TCP group (the control group). Therefore, there were 10 maxillary sinuses per group.
The inclusion criteria comprised good oral health, bilateral edentulism in the maxilla, age between 45 and 70 years, and the bone height of the alveolar ridge between the alveolar crest and the bottom of the maxillary sinus ranging from 0.5 to 5 mm.
The exclusion criteria were as follows: metabolic diseases that affect normal bone metabolism, such as hyperparathyroidism or osteoporosis; being treated with drugs that cause bone metabolic disorders, such as corticosteroids, oral contraceptives, hormonal or chemical treatment, without ever having undergone radiotherapy to the head and neck region; general systemic diseases, such as diabetes, cardiovascular disorders, leukemia, hypertension, and coagulation disorders; autoimmune diseases; and any local contraindications, including the inflammation of the maxillary sinuses.
Methods
Primary stage
A cone-beam computed tomography (CBCT) image was taken before the commencement of the surgical procedure. This phase was considered as time zero (T0). The dental scaling of the jaws with the use of chlorhexidine (0.12%) rinses was performed 2 or 3 days prior to surgery. The medication (a 750 mg Levoflox (levofloxacin) tablet) was prescribed 24 h before surgery and 9 days post-surgery.
Second stage
Immediately before the surgical procedure, advanced platelet-rich fibrin (A-PRF) was prepared by aspirating 60–80 mL of blood from the patient’s basilar vein in the elbow fold, using 20-milliliter syringes or 24-gauge intravenous catheters when a larger volume was required. The aspirated blood was placed in special A-PRF tubes and centrifuged immediately at 1,500 rpm for 14 min.14
Surgical method
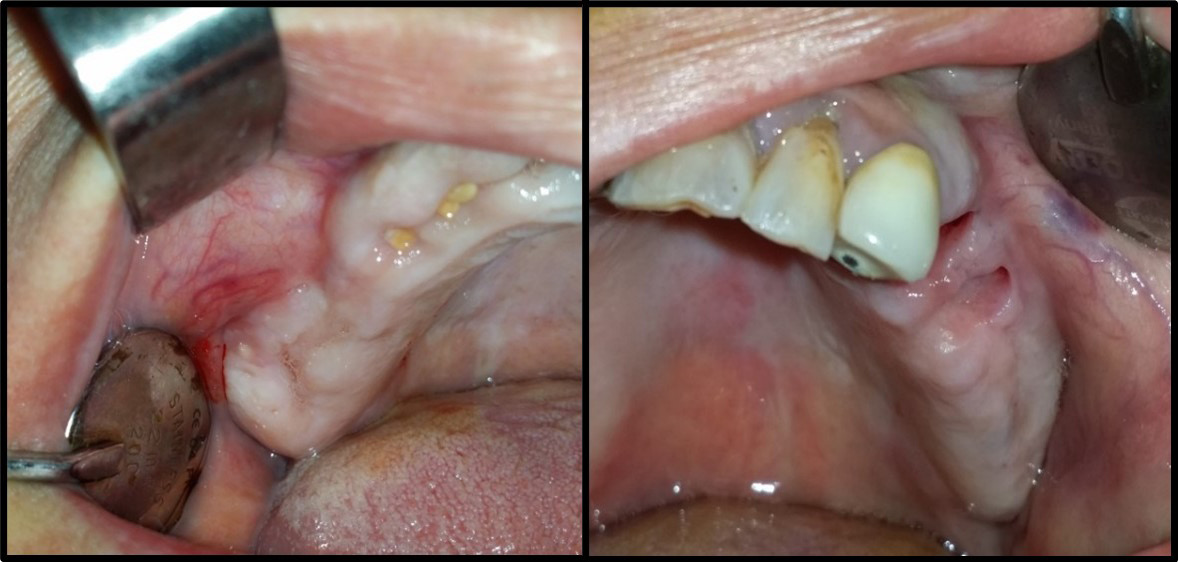
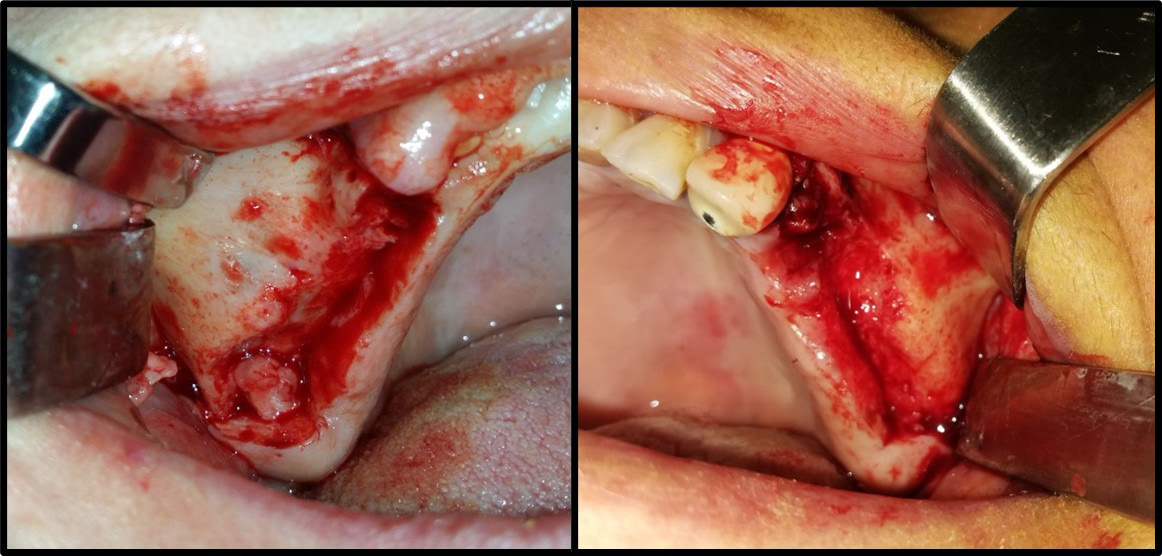
The mouth was disinfected with 0.12% chlorhexidine rinses, the skin around the mouth was disinfected with a polyvidone iodine solution and the surgical area was isolated using sterile surgical scrubs. Local (buccal and palatal) anesthesia utilized 2% lidocaine hydrochloride (HCl) and epinephrine (1:80,000) (Figure 2). A trapezoid-shaped, full-thickness mucoperiosteal buccal flap was then created (Figure 3).
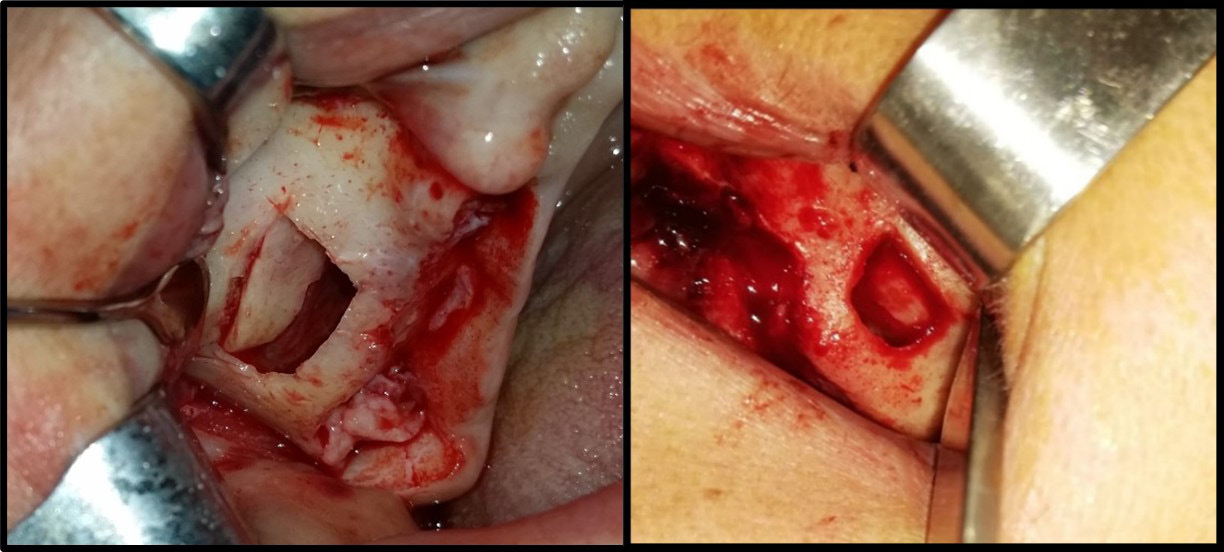
Using a piezosurgical device with appropriate saline irrigation, a bony window with rounded corners was created to reduce perforation during lifting. It had dimensions of 12–15 mm in length and 10 mm in height based on the size of the area to be grafted. A CBCT radiograph indicates the thickness of the bony window, which facilitates its preparation; 2–3 mm above the bottom of the maxillary sinus to enable sufficient vision during work and reduce the tension of the sinus membrane in the initial lifting phase. However, the window should not be enlarged much, as the surrounding walls aid bone healing (Figure 4).
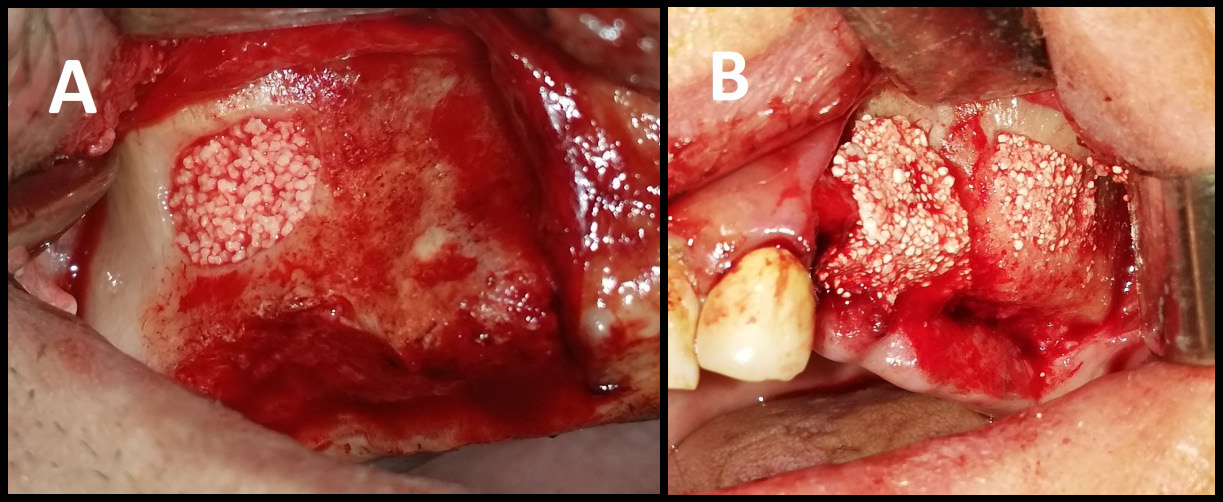
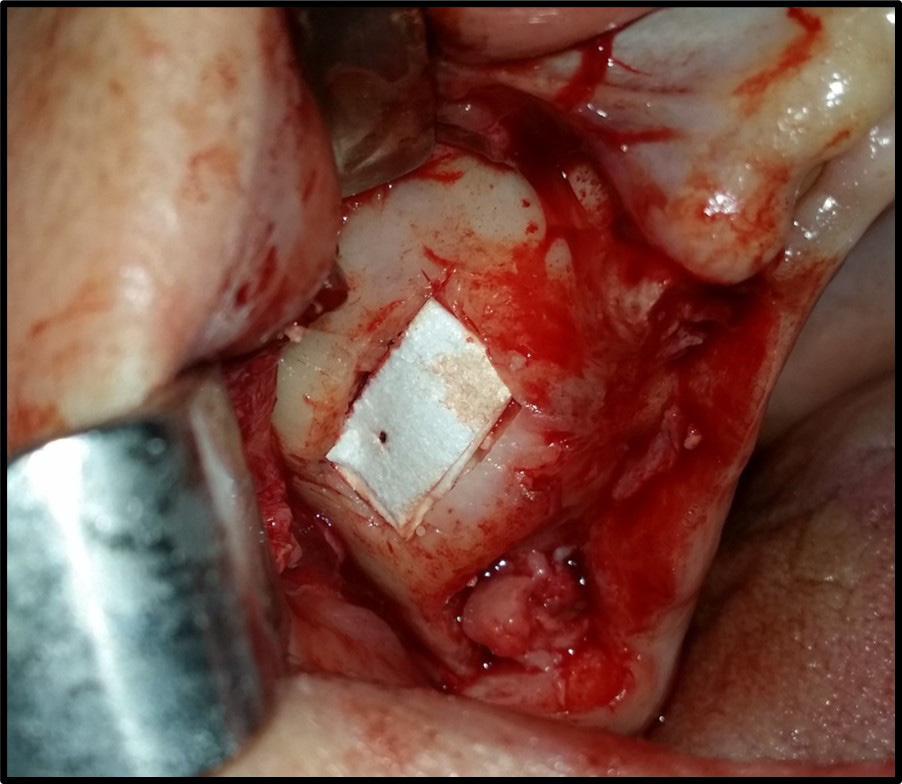
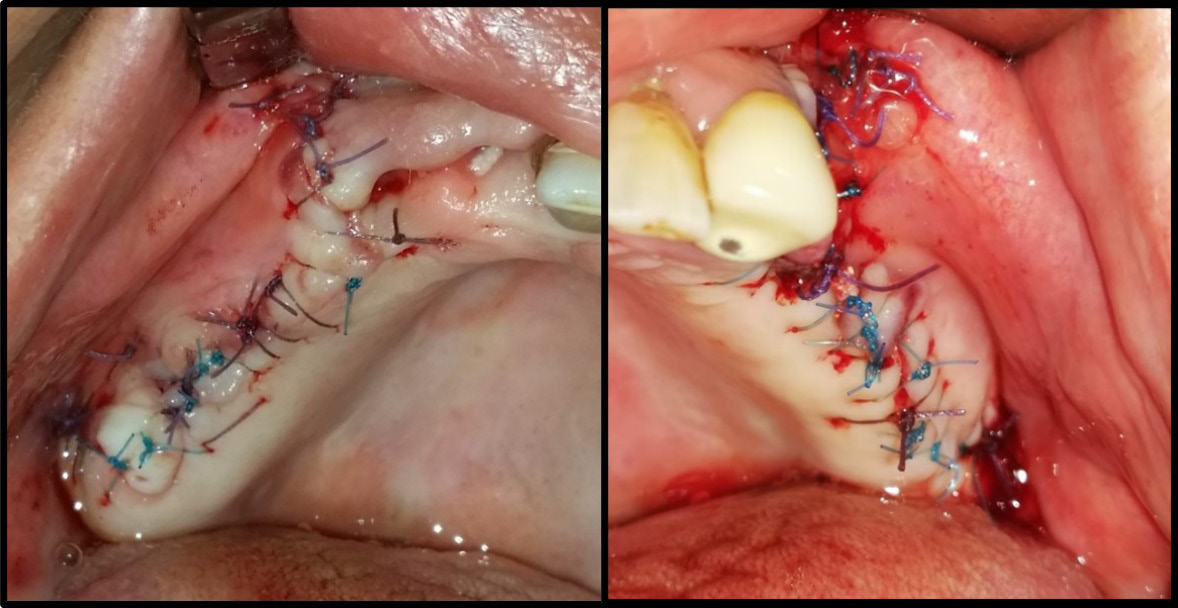
The sinus membrane was elevated with a sinus lift tool – the Dentium Advanced Sinus Kit (DASK) (Dentium, Cypress, USA), and for the grafting of the maxillary sinus, the mixture of CS or TCP with A-PRF was used (Figure 5). When using the TCP/A-PRF compound, we placed a collagen membrane on the bony window (Figure 6). The CS/A-PRF compound does not require any membrane, as a catalyst is added to the CS graft to harden fast. As such, the CS graft replaces the membrane due to its hardening and slow absorption properties.15
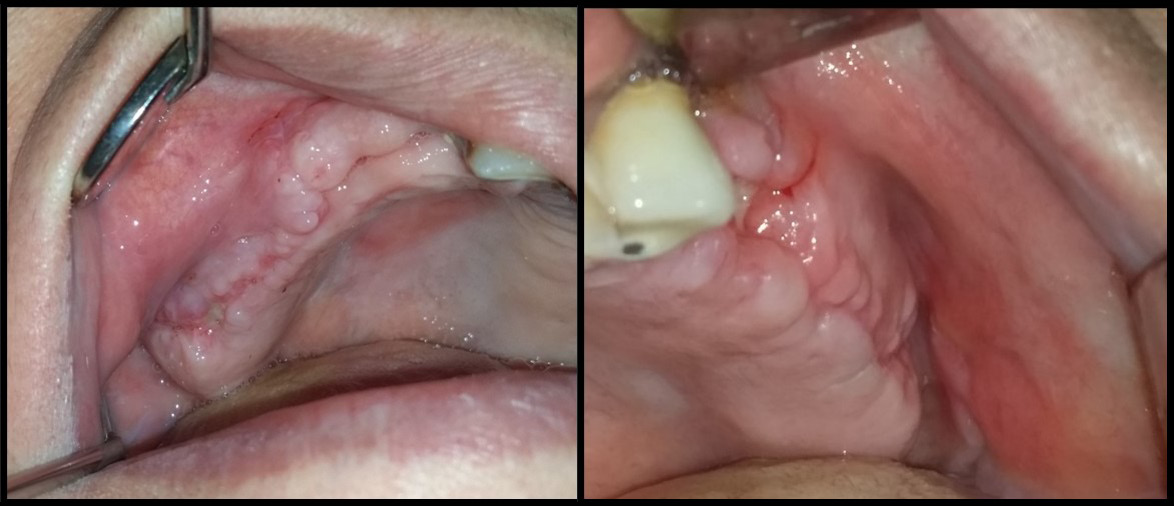
Interrupted suturing employed 4-0 Prolene sutures and a reverse cutting needle (Ethicon US, Cincinnati, USA) (Figure 7). The patients received post-surgery instructions and a medical prescription, and had a follow-up appointment to remove the sutures (Figure 8).
Radiological study method
Three CBCT radiographs were performed for each patient with the use of the PaX-i3D Green imaging system (Vatech, Hwaseong, South Korea). All scans were conducted in the same radiology center to standardize the characteristics of the radiographs, with the same position being repeated pre-op (T0), immediately post-op (T1) and 6 months post-op (T2), i.e., before the 2nd surgical operation (implantation).
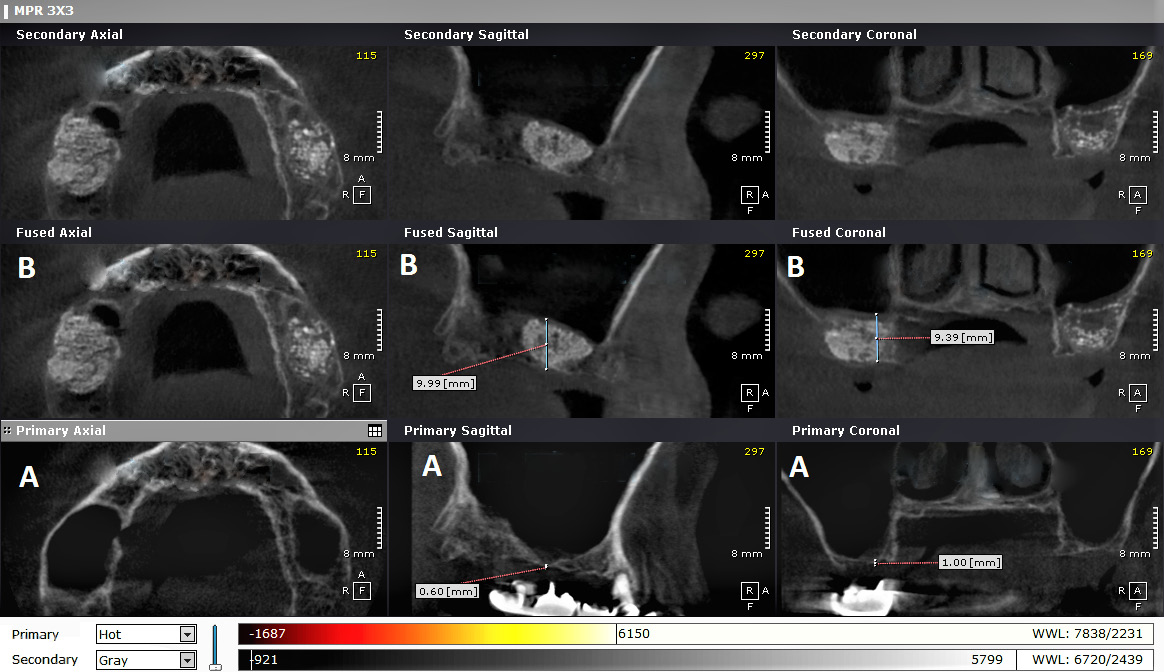
The radiographs were examined using the OnDemand3D program (https://www.ondemand3d.com/en), which enabled standardization by merging the 2 radiographs (taken at T0 and T1) to ensure the measurements of the same site on both scans, and to avoid any changes that could be caused by altering the position of the patient’s head (Figure 9).
The 1st image (T0) allowed the measurement of bone height before the maxillary sinus lift, using points in the sagittal view. The 3rd image (T2) was used to measure the amount of lifting at the same sites.
Bone height was measured on the 1st image (T0) at 5 locations in the coronal view, where each point in the coronal view matched the corresponding point in the sagittal view, using the ‘ruler’ tool. Bone height was also measured on the 3rd image (T2) at 5 locations in the coronal view. Using the same method, we measured bone height in the sagittal and coronal views on the 2nd image (T1).
The following measurements were calculated: bone height immediately after surgery; bone gain after 6 months; and bone reduction after 6 months (Equation 1,Equation 2):
Statistical analysis
The IBM SPSS Statistics for Windows software, v. 25.0 (IBM Corp., Armonk, USA) was used to perform all statistical analyses, and a p-value of 0.05 was considered statistically significant. The Shapiro–Wilk test determined the normality of data distribution, and the independent t test evaluated differences between the 2 groups at T0, T1 and T2.
The null hypotheses were as follows:
– There is no statistical difference between T0 and T2 in bone height in the TCP group (1).
– There is no statistical difference between T0 and T2 in bone height in the CS group (2).
– There is no statistical difference between the TCP and CS groups when comparing bone gain (3).
– There is no statistical difference between the TCP and CS groups when comparing bone reduction (4).
Error of the method
A total of 25% of the measurements were randomly selected and repeated a month after the 1st measurement by the same examiner (MAA). Systematic and random errors were calculated by comparing the 1st and 2nd measurements with the use of the paired t test. No statistically significant differences were found between the 1st and 2nd measurements for any variable (p > 0.05).
Results
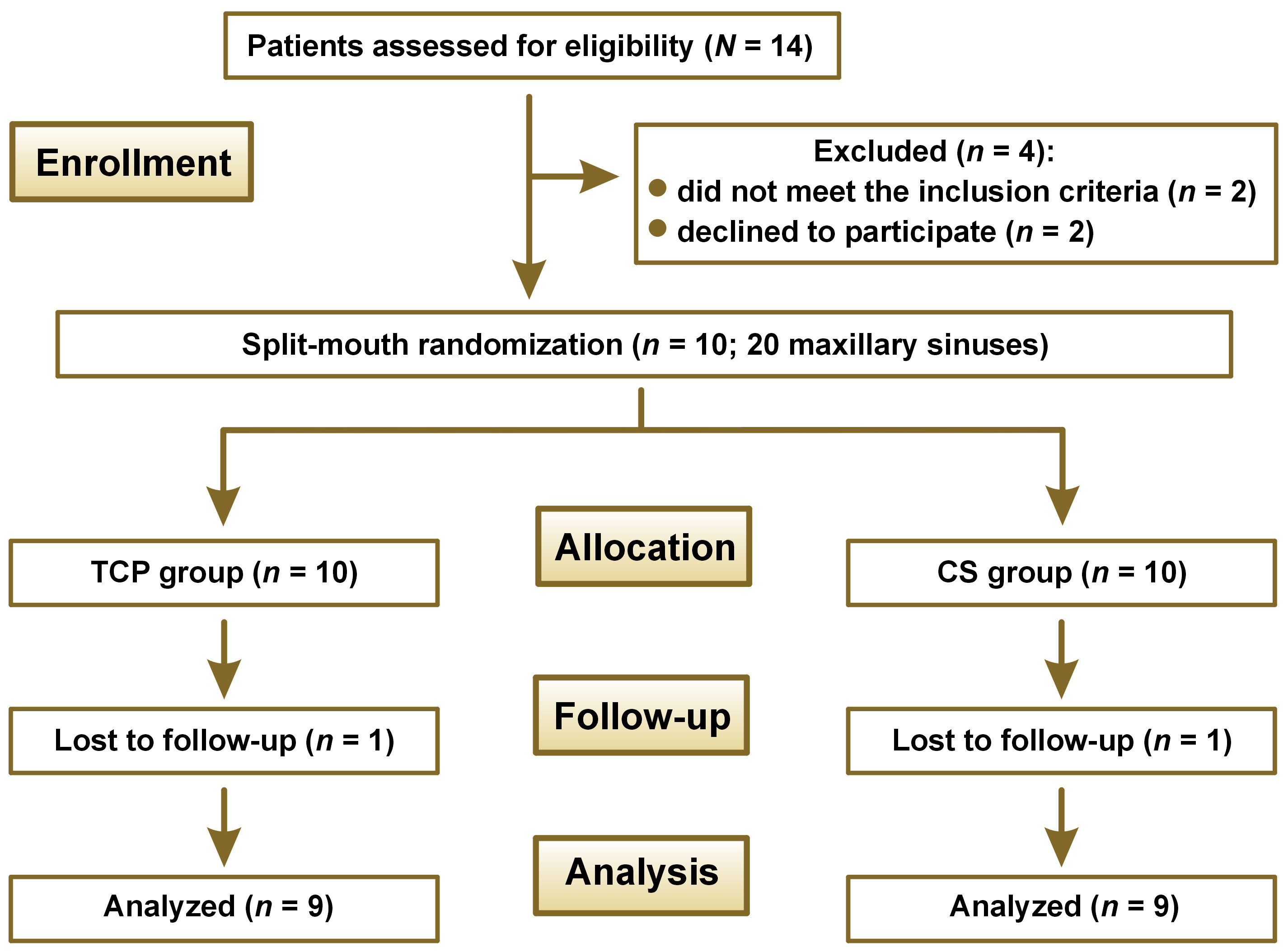
Figure 1 shows the CONSORT flow diagram. The study included 20 maxillary sinuses in 10 patients, though 1 patient was excluded from the sample after refusing to attend the radiographic follow-up. Therefore, a complete follow-up was done for 9 patients (18 maxillary sinuses), and the statistical analysis was conducted. The descriptive statistics of bone height in both groups at each time stage are shown in Table 1.
The results presented in Table 2 and Table 3 indicate that bone height increased significantly in the TCP and CS groups between T0 and T1 and between T0 and T2, and decreased significantly between T1 and T2 (p < 0.05).
Table 4 shows that there were no significant differences, at a confidence interval (CI) of 95%, in the amount of bone gain (p = 0.693) or bone reduction (p = 0.678) between the 2 groups. The mean bone gain in the CS graft was higher than in the TCP graft, with a minor difference recorded (0.43 mm). Also, the mean bone reduction in the TCP graft was slightly greater than in the CS graft, with a 0.44 mm difference between the two.
Discussion
Maxillary sinus lifting is a still evolving procedure, necessary to increase bone height for dental implants. However, performing the procedure requires extended knowledge of maxillary sinus anatomy and its variations, as there may occur difficulties in window preparation, causing the perforation of the Schneiderian membrane. Before sinus lift surgery, CBCT should be conducted to discover the prevalence of septa.16
Many studies have investigated the optimal bone graft for sinus lifting, although autogenous bone grafts are considered the gold standard, as they provide osteoconduction, osteoinduction and osteogenesis – the 3 essential elements for bone regeneration.16 However, the additional surgical site created to obtain an autograft increases the procedure time and causes more pain to the patient.16, 17 These factors have led to increasing interest in the search for alternative graft materials, mixing autografts with other types of bone grafts or completely replacing them with other grafts.9
There is increased interest in using alloplastic bone grafts to facilitate surgical procedures. However, the large amounts of materials required in cases of high maxillary sinus bone absorption increase expense. Therefore, studies have investigated mixing alloplasts with A-PRF to reduce the amount of graft needed and promote bone graft osteogenesis. Advanced PRF is an autologous graft material that eliminates any risk of disease transmission. In addition, its gelatinous consistency improves clot and graft stability, as it reduces the time required to ossify.11
Based on the current literature, the present research aimed to investigate the effectiveness of the CS/A-PRF compound in the grafting procedure after a maxillary sinus lift. Calcium sulfate is readily available, provides acceptable results, is easy to use, and helps to reduce surgical costs. Also, unlike other bone substitutes,18, 19 it can be used without absorbable and non-absorbable membranes.18
To verify the CS graft results, it was compared with TCP, as it is the same class of alloplast, it is reliable and is frequently used in the grafting procedure,6 and provides a resorbable scaffold for bone growth.9 Both grafts were mixed with A-PRF to accelerate and increase new bone formation, and reduce the amount of graft material used.11 Since there are no previous studies in the literature comparing these 2 grafts, this study aimed to compare CS/A-PRF with TCP/A-PRF in the external sinus lift procedures, using the radiographic measurements of bone gain and bone reduction after 6 months.
The study sample included 20 maxillary sinuses of 10 patients who had a bilaterally edentulous posterior maxilla, were aged 45–70 years, had bone height between the alveolar bone crest and the bottom of the maxillary sinus ranging from 0.5 mm to 5 mm (class SA4 according to Misch classification), did not suffer from any systemic diseases affecting the surgical procedure, and did not suffer from any health problems in the nose and sinuses that are considered a contraindication for sinus lifting. One patient was excluded from the sample, because he refused to attend the radiographic follow-up. Therefore, the sample consisted of 18 maxillary sinuses (9 patients) randomly distributed into 2 groups, with 9 sinuses in the CS group and 9 sinuses in the TCP group. A split-mouth technique was used, where the CS/A-PRF graft was applied to one maxillary sinus, and the TCP/A-PRF graft was applied to the other side.
The lateral approach technique was followed to lift the maxillary sinus, since this method is indicated for maxillary sinus elevating in class SA4 cases and the delayed implantation excludes other methods, such as the alveolar approach (the internal sinus lift).20 In addition, the implant success rate is higher in the two-stage method than in the one-stage technique in SA4 cases.21
An ultrasonic system was used for window preparation, similar to other studies,22 as it reduces the incidence of the perforation of the maxillary sinus membrane to 7% from the 25% experienced with rotary instruments. The method also reduces pain, discomfort and edema after surgery, and generally helps to protect soft tissues, including the maxillary sinus membrane and the mandibular nerve.23
After maxillary sinus grafting, the bony window was covered with an absorbable collagen membrane on the side grafted with the TCP/A-PRF compound, which helps to prevent the surrounding connective tissue cells from entering the bone graft material and increases vital bone formation.24 No membrane was used for the CS/A-PRF compound, and the window was covered only with the CS graft material. The material can be used as a membrane due to its physical properties,15 which reduces the financial cost and the surgical procedure time.
The radiological study used CBCT images, which are accurate and sufficient for determining reference points, and three-dimensional (3D) measurements can be made with ease. Three radiographs were performed for each patient to study bone gain and bone reduction in the grafted area,25 with the 1st one taken pre-op (T0), the 2nd immediately post-op (T1) and the 3rd 6 months post-op (T2).
The CS/A-PRF graft material proved to be useful and safe for the two-stage external maxillary sinus lifting procedure, as bone sufficient for dental implant placement was obtained after 6 months. The mean bone height at T0 was 3.545 ±2.131 mm, which increased immediately to 13.858 ±1.966 mm at T1. At T2, bone height decreased to 11.506 ±2.440 mm, giving a gain of 7.961 ±2.781 mm and a reduction of 2.352 ±1.832 mm.
Guarnieri et al.8 and Tarnow et al.26 reported that CS grafts promoted implant stability and new bone formation after its absorption. However, a study that followed up 2 years after the 1st surgery found a greater reduction than the current study (1.0–3.5 mm), perhaps due to the difference in the observation period (2.5 years).15
The use of TCP/A-PRF as a grafting material after external sinus lifting helped to secure a sufficient amount of bone for implantation, where the average bone height at T0 was 3.859 ±1.728 mm and it increased immediately to the size of the graft at T1 (14.185 ±3.025 mm). After 6 months, bone height decreased to 11.391 ±0.934 mm, resulting in a gain of 7.532 ±1.150 mm and a reduction of 2.794 ±2.310 mm. Oba et al. recorded a gain of 3.11 ±1.35 mm, which is less than in this study, perhaps because their study used the osteotome sinus lifting technique,27 which does not allow to achieve a high bone gain as compared to the lateral lifting method.25 Okada et al. also found a reduction of 0.73 ±1.33 mm, which was due to the differences in the radiological and surgical methods, where the implants were placed at the same time as grafting.28
When comparing the 2 study groups, we did not find significant differences in the amount of bone gain or bone reduction, and no clinical or radiological complications were observed during the 6-month follow-up period.
Conclusions
Both grafts can be used for maxillary sinus lifting with the delayed implantation. The properties of CS are negatively affected by moisture, so the graft must be applied with a pasty texture in addition to isolating the receiving area, and it is better to apply the material in layers to reduce shrinkage. The A-PRF material helped to increase the graft size, reduced costs and promoted bone formation, while it also helped to increase TCP graft bonding.
Ethics approval and consent to participate
Informed consent was obtained from the participants, and the ethics board at the Faculty of Dentistry of Damascus University, Syria, approved the study (FMD-185).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.