Abstract
Platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) are biological products derived from the plasma fraction of autologous blood that have a platelet concentration above that of the original blood. Cytokines and growth factors are present in platelet-based preparations, and their application has gained great attention in dentistry. The aim of this review was to comprehensively examine the latest scientific evidence on the use of PRF and PRP in oral surgery, and to describe current operational protocols. Platelet-rich fibrin is used after third molar extractions, in the treatment of alveolar osteitis and trismus, and in implant surgery. Platelet-rich plasma is utilized in sinus lift procedures, after tooth extractions, and in patients undergoing the treatment of bisphosphonate-related osteonecrosis of the jaw. Based on this review, plenty of data indicates that the PRF-PRP usage in oral surgery shows promising results. However, no consistent protocols have been presented in the analyzed articles. Further research is needed to provide clinicians with evidence-based clinical recommendations and to develop protocols on the use of these preparations in dental surgery.
Keywords: dental surgery, platelet-rich plasma, platelet-rich fibrin, operative protocols
Introduction
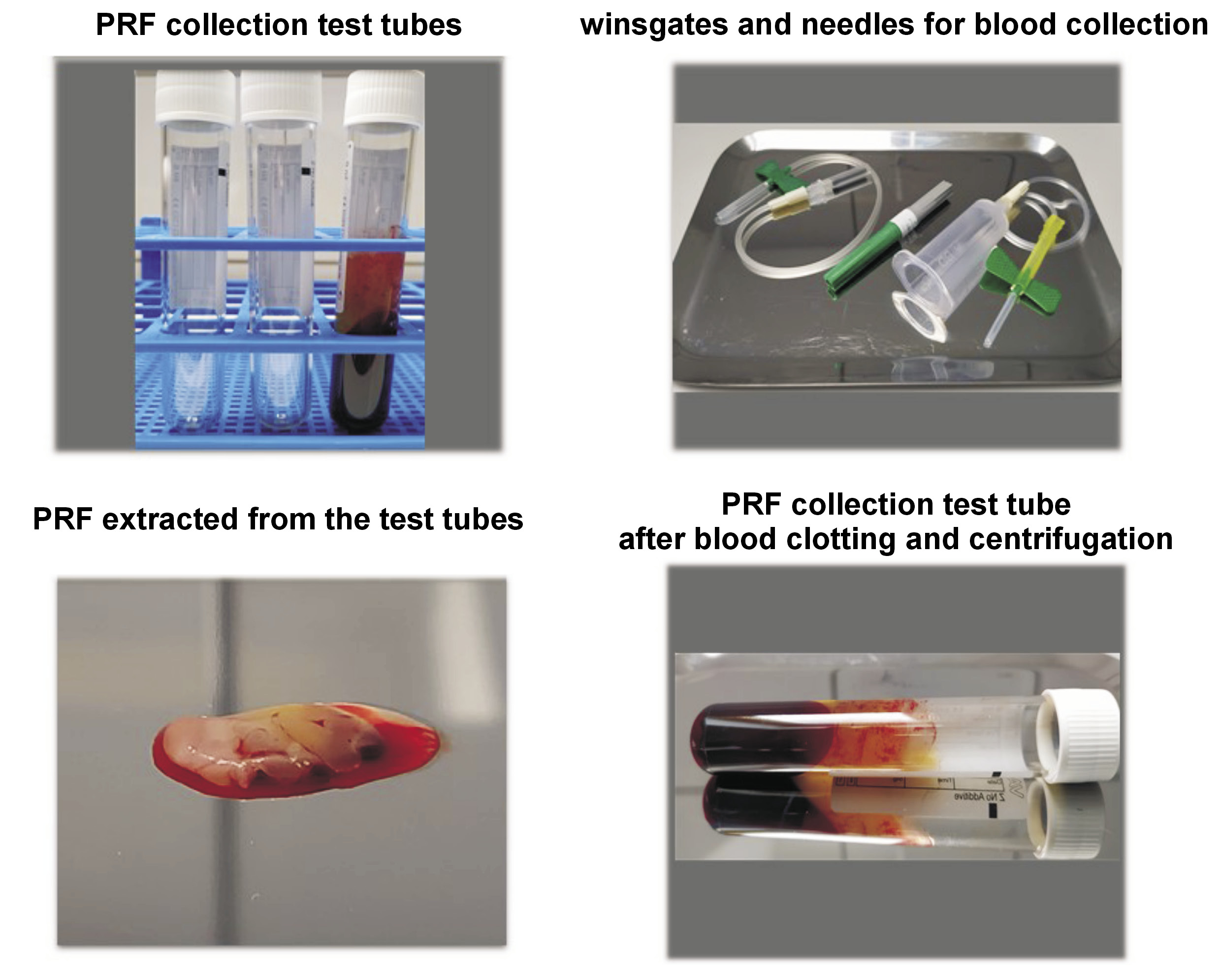
In terms of the general use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in the field of dentistry, these biological products are widely used in oral surgery.1, 2, 3 The potential of these biomaterials in dentistry is based on the presence of cytokines and growth factors in platelet-based preparations that support the healing process.1, 4 There is, however, no consistency in the laboratory preparation of these materials, and clinical protocols for the use of PRP and PRF in dental patients are diverse (Figure 1).3
The aim of this article is to present the latest reports on the use of PRF in oral surgery, which is currently a subject of interest to the medical community. In recent years, many questions have been asked about the relevance of using PRF in several dental surgical procedures, including third molar extractions, dental implantology, and in patients undergoing bisphosphonate (BP) therapy. This review intends to synthesize the current knowledge and show the possible advantages and limitations of the use of PRF during surgical procedures in the maxillofacial region.
Methods
PubMed and Google Scholar were searched using the terms “platelet-rich plasma”, “platelet-rich fibrin”, “PRF”, “PRP”, AND “oral surgery”. The abstracts resulting from the searches underwent an initial review to see whether they fit with the search requirements. Articles were selected based upon their relevancy to general medicine, prioritizing original works, case reports, clinical trials, and clinical practice guidelines based upon the quality of the journal and the authors’ experience in the area.
Types of platelet concentrates
In 2009, Dohan et al. proposed a classification system for platelet concentrates (PCs) based on the method of preparation, differences in content, and the properties of the received fibrin network.5 The authors distinguished four groups of PCs:
– pure PRP/leukocyte-poor PRP (PPP/P-PRP);
– leucocyte and PRP (L-PRP);
– pure PRF/leucocyte-poor PRF (P-PRF); and
– leucocyte and PRF (L-PRF).
The properties of each concentrate are dependent on the preparation technique and are presented in Table 1. Table 2 presents the comparison between some clinical and laboratory parameters used in surgery research with PRF.
Use of PRF after mandibular third molar surgery
Removal of the third molars is one of the most frequently performed procedures in oral surgery.1, 4 PRF is widely used to facilitate the healing process after tooth extraction, as postoperative wound healing is a multi-stage complex process that aims to re-establish tissue integrity and functional efficiency.6 Although PRF is commonly used after third molar extractions, the outcomes in terms of post-surgical difficulties remain unknown, and randomized controlled trials to vindicate its use are lacking.1, 7, 8, 9
Influence of PRF on postoperative pain and swelling
In a study conducted by Singh et al., 20 patients underwent bilateral extraction of the third molars followed by the application of PRF into only one extraction pocket (study site), while the other site was left untreated (control site).10 The results showed that there was less pain and that soft tissue healing was better in the PRF pocket.10
Ozgul et al.11 performed a study on 56 patients after bilateral third molar extractions, followed by the application of PRF to only one pocket (the same process as in the study performed by Singh et al. cited above).10 It was reported that, on the first and third days post-surgery, horizontal swelling, measured from the tragus to the commissure, was significantly decreased. However, in the work of Gülşen and Şentürk performed on 30 patients, the results showed no statistically significant difference in post-surgical pain between the study and the control groups.12
Trybek et al. performed a study involving 90 patients with impacted lower third molars.13 All surgical procedures were performed under an antibiotic cover of 600 mg clindamycin administered one hour before the surgery. The results showed that patients from the study group (with PRF application in the extraction pocket) reported a lower pain intensity at 6 hours, 1 day, and 3 days after surgery. In addition, body temperature was significantly higher in the control group than in the study group on day 2 postoperatively. The authors also observed that trismus was significantly lower in the study group than in the control group at 1, 2, and 7 days after surgery. However, PRF application did not significantly affect the intensity of swelling. The authors claimed that the application of PRF may lead to less traumatic treatment and faster recovery.
Impact of PRF on alveolar osteitis
Alveolar osteitis (AO) is observed in 0.5–5% of patients after routine dental extractions.14 However, the incidence of AO following the extraction of mandibular third molars is reported to range from 3.9–29.6%.15, 16
Yüce and Kümerik conducted a study of 40 patients with positively diagnosed and untreated AO within 3 days after extraction of a mandibular third molar.15 The participants were divided into two groups of 20 patients. The patients from the control group had their extraction pockets curetted and rinsed with saline, while the patients from the study group underwent the same procedure and had PRF applied to the pockets. All patients were examined on days 1, 3, 7, and 15, and 1, 2, and 3 months after the procedure. The soft tissue healing process was evaluated postoperatively using the Wound Healing Index of Landry (Turnbull and Howley). There was a statistically significant difference in the timing of the epithelialization process observed between the groups. The healing rates were significantly faster in the PRF group compared to the control group on every day of examination. Pain was evaluated using the Visual Analogue Scale, and it was found that the pain scores in the study group on the first, third, fifth, and seventh postoperative days were significantly lower than in the control group. It was concluded that the application of PRF might improve and accelerate the therapeutic process of tissue regeneration and may have a positive effect on pain reduction in the management of AO. In addition, the trials conducted by Eshghpour et al.17 and Al-Hamed et al.18 both confirmed a significant decrease in the occurrence of AO after the application of PRF to the extraction pocket.
Osteoblastic activity after PRF application
Currently, two studies have evaluated the effects of PRF on osteoblast activity.1 According to Baslarli et al.19 and Gürbüzer et al.,20 there were no statistically significant differences in the activity of osteoblasts between the case (extractions followed by application of PRF) and the control groups (traditional extractions). In both studies, the results were estimated using bone scintigraphy based on the uptake of technetium-99m methylene diphosphonate in the extraction pocket.
Effect of PRF use on trismus
Trismus is a spasm that is a frequent problem in oral and maxillofacial surgical practice. The causes of this condition may be generally classified as articular or extra-articular. Shires et al. have defined trismus as a lengthened tetanic spasm of the masticatory muscles, which restricts mouth opening.21, 22 However, it is often used as a synonym for a decreased range of mouth opening ascribed to extra-articular causes.23 Trismus is one of the most common complications that occur after removal of mandibular third molars.24, 25
Uyanik et al.26 initially performed 40 extractions of impacted mandibular third molars in 20 patients, and repeated their study in 2016 on a group of 56 patients (21 bilateral extractions and 38 unilateral extractions).27 Both studies showed statistically significant differences in the extent of trismus between the study group (extractions followed by application of the PRF) and the control group (traditional extractions), only on day 1 after the surgery. They did not observe any statistically significant differences between groups on later days.
In sum, recent studies have shown a positive effect of PRF in reducing postoperative pain and swelling, and decreasing the incidence of AO. However, the beneficial effects of PRF on osteoblastic activity, trismus, and soft tissue healing have not been clearly demonstrated and require further research.1, 7
Use of PRF in dental implantology
Immediate dental implants
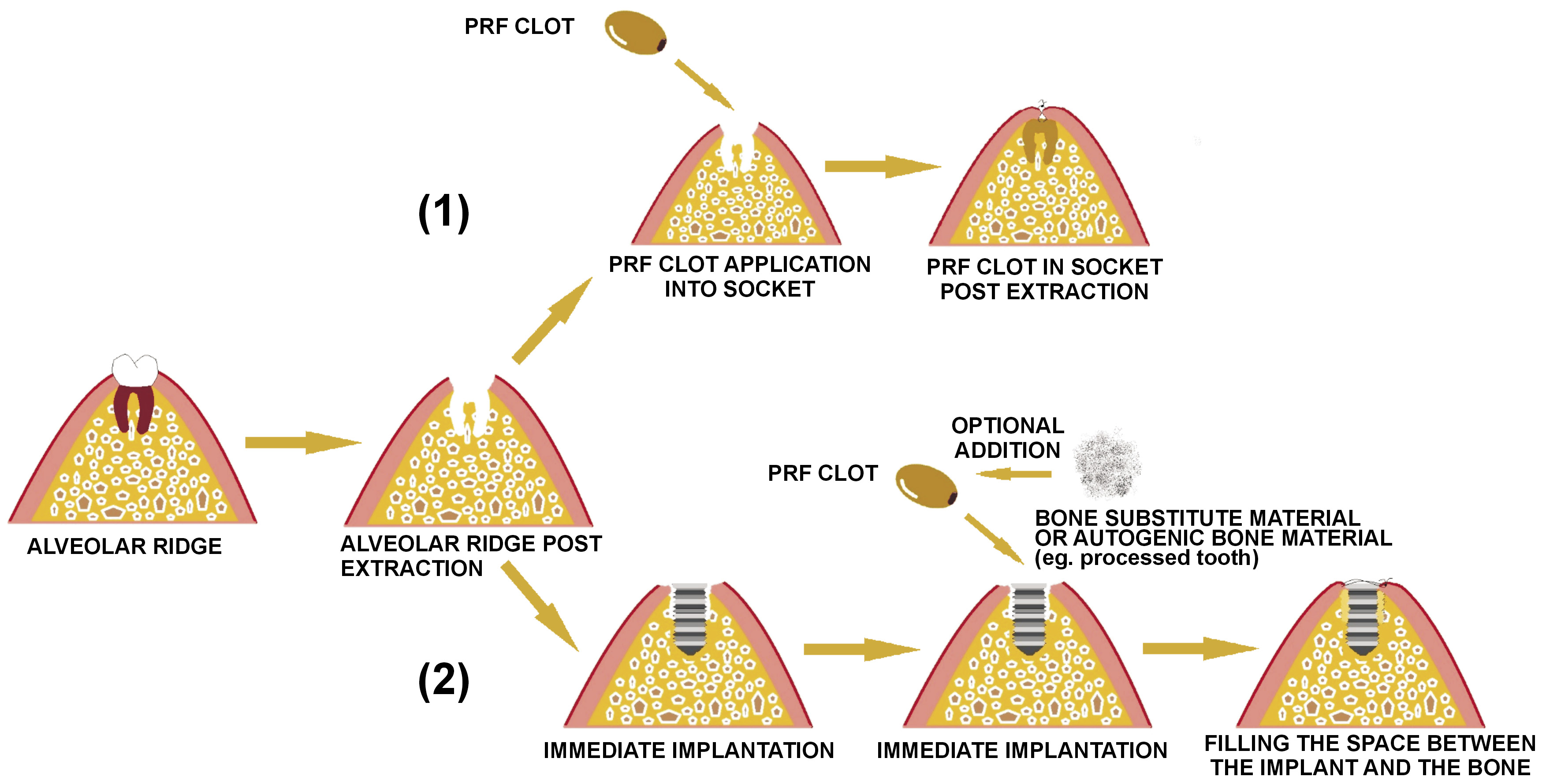
Over the years, immediate implant placement has gained popularity due to its numerous advantages (Figure 2).28, 29, 30 It has been shown that this method minimizes the period of the treatment and the number of surgical visits, which has a positive effect on the patient’s comfort.31, 32 Some concerns have been reported regarding immediate implant placements in the molar area, and it has been suggested that large molar roots may result in an unsatisfactory bone quantity. To overcome this, the standard procedure is guided bone regeneration, which leads to an augmentation of bone around the implant. The literature reports that PRF is a useful source of growth factors, such as bone morphogenetic protein (BMP), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor (TGF) , and that it allows the regeneration of adjacent tissues.28 In addition to this, PRF, being an autologous biomaterial, stimulates cell proliferation, migration and angiogenesis, and prevents infection.33, 34
After an extraction, the wound area goes through many physiological processes, such as bone resorption and gingival remodeling.35, 36 It has been proposed to place an implant soon after the extraction to protect the osseous complex. Healing of the bone next to the implant starts with the formation of a fibrin clot. The implant surface activates the platelets stuck to the fibrin. Other authors have claimed that platelets are an excellent source of the previously mentioned growth factors, and that the application of PRF leads to better bone regeneration and faster osseointegration of titanium implants.37, 38
Öncü E et al. studied 26 patients (16 men and 10 women) who underwent 60 immediate implantations (study group: 30 implantations with a PRF clot, control group: 30 implantations without a PRF clot).37 The procedure consisted of a crestal incision followed by luxation and extraction of the teeth. Following this, the pockets were cleaned, and the granulation tissue was removed. Subsequently, the areas for the implants were prepared, and PRF was applied to one of the implant pockets. In the other pocket, the implant was inserted without a PRF membrane. The flaps were sutured after bringing them back to their original position. Changes in bone loss on periapical radiographs were observed 7 days, and 1, 3, and 12 months after surgery. Resonance frequency was also measured using the Osstell® device, which establishes the stiffness of the bone–implant complex. The implant stability quotient measurements were expressed as numerical values from 1 to 100.38 The results showed that the stability after 1 week and 1 month was significantly higher for the test group. In addition, the difference in mean marginal bone resorption was significant between groups (0.5–0.7 mm for the test group and 0.6–1.3 mm for the control group).
Peri-implantitis
Studies on peri-implantitis have shown that peri-implant complications are not rare, and the fact that an implant survives does not always mean that it was a successful implantation.39, 40 Peri-implant diseases may manifest in two forms: peri-implant mucositis, which is a lesion in the soft tissue around the implant with no signs of bone loss but with bone remodeling, and peri-implantitis, which causes bone loss.41, 42, 43 Various treatment strategies have been proposed for this condition, including pharmaceutical therapy, mechanical debridement, and surgical procedures (e.g., decontamination, smoothing the implant surface, resection, or bone regeneration).44, 45 Modern medicine also allows for the opportunity to use PRF.46
Boora et al., in their study on the influence of PRF on peri-implant soft tissue and crestal bone level in a one-stage implant placement procedure, reported heterogeneity in their results.47 In this study, 20 patients were divided into two groups of 10 people each. The test group had their implants placed with an application of L-PRF, and the control group had theirs placed without it. The participants underwent clinical and radiographic examination at the time of implant placement, and at 1 month and 3 months postoperatively. Less initial marginal bone loss was observed in the test group at the mesial and distal sites of the implants at 1 and 3 months. However, there were no statistically significant differences in pocket probing depth or bleeding on probing between the test and control groups at all time points. The implant survival rate was 100% in both groups.
Hehn et al. examined the effects of PRF on soft-tissue thickening and initial marginal bone loss around implants.48 This study involved 31 patients, each of whom underwent an implantation procedure in the lower mandible using a split-flap technique. Ten implants were placed with soft tissue augmentation using a L-PRF membrane (test group), and 21 implants were placed without the use of L-PRF (control group). Tissue thickness was measured at the time of implant placement and at 3 months follow-up. Radiographic evaluation was carried out at the time of implant placement, and at 3 and 6 months postoperatively. A statistically significant thickness loss at the crest was observed in the PRF group compared to the control group. There was no statistically significant difference in bone loss observed between the mesial and the distal sides in the control group. The authors concluded that soft tissue augmentation with a L-PRF membrane combined with the split-flap technique cannot be recommended for the thickening of thin mucosa.
Role of PRP in sinus lift procedures
Resorption of the maxilla is becoming a more common clinical condition and requires patient-specific procedures that allow for a reduction in intraoperative timing and maximization of postoperative compliance.49, 50 Focal or generalized atrophy may be caused by multiple factors, but edentulism plays a primary role.49, 51, 52
The idea of maxillary sinus floor augmentation was first described by Tatum (1977) and first published by Boyne and James (1980). In many cases, the procedure is essential to achieve correct placement and positioning of an implant.53
In 2012, Tatullo et al. performed a study on 60 patients to investigate, both clinically and histologically, the potential use of PRF combined with deproteinized bovine bone as grafting materials in a sinus lift for severe maxillary atrophy.49 The results were based on comparisons with a control group, which received only deproteinized bovine bone as a grafting material. In this study, a total of 72 sinus lift procedures were performed. Twelve patients had bilateral atrophy of the maxillary cortex, and the procedures were performed on both of their sinuses, with each of them being applied differently. One side served as the control group and the other as the study group. In addition, patients were divided into three groups according to the length of time between the sinus lift procedure and implant surgery (106 days, 120 days, and 150 days). Before placement of the implant, a transcortical bone sample was obtained from the area of the performed sinus lift procedure, which was subjected to histological and histomorphometric analyses. All treated cases were successful, including both the reconstructive surgery and subsequent rehabilitation with implants. Histological analysis showed that the samples treated with PRF that were collected after 106 days consisted of lamellar bone tissue with interposed stroma that appeared richly vascularized. These results suggest that PRF reduces healing time and accelerates the process of bone cicatrization. According to the authors, it is possible to obtain good stability of endosseous implants placed 106 days after a sinus lift procedure.
A similar study was conducted by Gurler et al.54 This trial included 28 patients, but data from only 24 were evaluated. The patients were divided into two groups: a control group that had a sinus lift procedure performed using only an allogenous bone graft, and a study group that additionally had a L-PRF membrane used to close a lateral window created during surgery. The results showed a slight improvement in postoperative complications, such as pain, swelling, and the quality and possibility of sleep and eating, in the L-PRF group; although the differences were not statistically significant (p > 0.05).
Comparable results were obtained by Cömert et al.55 and Gassling et al.53 Both of these studies showed that the use of PRF in sinus lift procedures did not have a statistically significant impact on the success of the surgery, and indicated that further evaluation is needed.
Administration of PRF
to patients undergoing bisphosphonate therapy
Bone metabolism disorders, such as osteoporosis and bone metastasis, require therapy with the use of BPs, which are bone-antiresorptive agents.56, 57 Their mechanism of action involves inhibiting osteoclast functions. However, BPs also have a negative influence on fibroblasts and osteoblasts in terms of disabled proliferation.57, 58, 59 There are reports that show a significant correlation between the use of these drugs and an increased risk of osteonecrosis of the jaw, particularly after a local injury (e.g., a tooth extraction).60, 61, 62
Tooth extraction
Pispero et al. conducted a study in which a 70-year-old woman needed observation of the upper right second premolar (15) tooth due to a suspected fracture.56 In the medical history, it was reported that she was on an alendronate based therapy for 12 years (one 70 mg tablet per week). The patient went through a professional hygienization a week before the planned surgery. On the day of the extraction, 20 mL of blood was taken from the patient and centrifuged to obtain a PRF clot. Anesthesia and atraumatic extraction of the root of the 15 teeth were carried out. Following this, the pocket was rinsed with a sterile saline solution and closed with two layers of PRF membrane (one in the alveolar pocket and the other above the alveolar pocket). It was decided to continue antibiotic therapy for a further 14 days, along with the application of a 1% chlorhexidine gel to the surgical area three times daily. When the mucosa had regenerated at the end of the second week after the surgery, the sutures were removed. Two months later, at a follow-up visit, no signs of inflammation or exposed bone were reported.
Scoletta et al. carried out a follow-up study that included 63 patients with 202 extractions performed.63 All of the patients had a history of intravenous therapy with BPs for at least 2 months. Minimally invasive extractions were performed under antibiotic cover (amoxicillin with clavulanic acid 600 mg, 3x per day for 6 days). Post-extraction, the alveolar sockets were cleaned with ultrasonic surgical devices. The pockets were filled with autologous plasma rich in growth factors (PRGF) and sealed with autologous fibrin. At the follow-up, the oral mucosa showed complete healing in most patients (98.41%) and did not differ from that expected in healthy patients. Computed tomographic scans showed normal alveolar bone healing. At the most recent follow-up visit, all patients had unimpaired mucosa and no signs of inflammation. The authors pointed to the significant differences in the duration of the surgical procedures between the present and previous protocols (the previous protocol used a vestibular flap.) Surgical time proved significantly shorter using the current approach.
PRGF may be an important factor in the successful treatment of patients undergoing BP therapy to restore the osteoblast–osteoclast homeostatic cycles via autologous cytokines.64 Moreover, PRGF can be helpful in shortening the time for recovery from surgical procedures while ensuring good treatment results.63 It seems that patients who have undergone BP therapy may benefit significantly from using autologous concentrates during surgical procedures, but this subject needs further research and more randomized trials.
Bisphosphonate-related osteonecrosis
of the jaw
Kim et al. studied the utility of PRF for the treatment of bisphosphonate-related osteonecrosis of the jaw (BRNOJ).65 This study included a total of 34 patients. All patients were at first treated conservatively with antibiotics, analgesics, an antibacterial mouth rinse, and daily irrigation with 0.12% chlorhexidine. Surgical protocols included the complete resection of all infected and necrotic tissues, intensive irrigation with antibiotics, the application of L-PRF, and primary closure. The results showed a complete resolution in 26 patients (77%), while 6 (18%) had a delayed resolution, and 2 (6%) showed no response to the treatment. The authors indicated that the treatment of BRONJ with PRF is very promising. However, they emphasized the need for further research using randomized prospective trials.
Gönen et al. performed a study on a 77-year-old male patient who complained about pain and swelling on the left side of his face.66 In the medical history, it was reported that the patient had prostate cancer and was receiving zoledronic acid for the treatment of secondary hypercalcemia due to the malignancy. Stage 3 BRONJ was diagnosed. Minimal sequestrectomy down to freshly bleeding bone was performed, and a thin layer of necrotic tissue was left to protect the inferior alveolar nerve. Due to the lack of acceptable gingival tissue to close the operative area, a PRF membrane was used to cover the wound. Two layers of PRF were obtained from the patient’s blood. In addition, an acellular plasma injection was performed around the wound. In the second week after surgery, epithelialization was observed with no infection or inflammation. In the fourth week postoperatively, new gingival tissue was reported. There was no paresthesia observed. Three months later, total coverage of the bone with new gingiva formation was accomplished. The follow-up lasted for 18 months and no exposure or recurrence was observed. The authors suggested that PRF may be an effective tool for closing exposed bone and promoting tissue healing in patients with BRONJ. However, this subject will need further research on a larger group of patients.
Discussion
PRF and PRP are currently widely used in oral surgery.1 Third molar surgery is the most common procedure using PRF1, 4, 7, and researchers continue to study its influences on postoperative pain and swelling.13 Recent studies have shown potentially successful outcomes following the use of PRF in extraction pockets, including a reduction in pain and swelling, a lowering of body temperature, and reduced trismus after surgery10, 11, 13; although other clinicians have not observed significant differences.12 There are also studies confirming that the healing process after PRF application may be faster in patients with AO.17, 67 Significant decreases have also been reported in the extent of trismus after third mandibular molar surgery followed by PRF application26, 27; although, there are insufficient studies on this topic. It has not been confirmed whether the use of PRF has an impact on osteoblastic activity after extraction.19, 20
PRF is widely used in the dental implantology field because of its delivery of growth factors that stimulate cell proliferation and angiogenesis.28, 33 It has been shown that PRF application immediately after implantation significantly increases implant stability and decreases bone resorption.37 There have also been studies on the use of PRF in peri-implantitis treatment, but the results are not consistent and more research on this topic is needed.47, 48
The application of PRF may be promising in the sinus lift procedure. Studies have shown a reduced healing time, rich vascularization, and faster bone cicatrization.49 One study has suggested that postoperative pain and swelling may be minimized by using a PRF membrane; however, the observed differences in this study were not statistically significant.53, 54, 55
There is a significant correlation between using BPs and an increase in the risk of osteonecrosis of the jaw. Researchers are still looking for ways to avoid this complication.60, 61, 62 Several studies have provided encouraging results for the use of PRGF in patients undergoing BP therapy. In the overwhelming majority of cases, proper healing of the mucosa and no bone exposure after dental extraction were observed in the treatment group.56, 63 It has also been demonstrated that this surgical approach may shorten the time for recovery from surgical procedures compared to traditional surgical extraction in patients at risk of BRONJ.63 Furthermore, the application of PRF may be helpful in the treatment of BRONJ. PRF stimulates the creation of new gingival tissue, decreases the time for healing, and may be a useful tool to help to close the wound after sequestrectomy. However, this topic still requires further studies that incorporate a large group of patients.
Conclusions
Currently, there are many case studies and meta-analyses on the use of PRF in oral surgery. The literature mostly favors the use of PRF and numerous studies have shown promising results. However, this subject needs further research because of the limitations in the previous work. Presently, there are no standard PRF protocols. In order to make the research more reliable, a single standard protocol should be created. It is certain that the successful use of a PRF product depends on the clinician’s skill and their understanding of the preparation technology. The growing popularity of centrifuges and the simplicity of the procedures at the dental office present an opportunity for the more widespread use of PRF in oral surgeries.
Ethics approval and consent to participate
Not applicable.
Data availability
All data generated and/or analyzed during this study is included in this published article.
Consent for publication
Not applicable.