Abstract
Background. Laser protocols for the treatment of dentin hypersensitivity (DH) have not yet been studied systematically.
Objectives. The present study aimed to review clinical trials on the treatment of DH with laser therapy through a systematic review and meta-analysis.
Material and methods. The search of electronic databases resulted in 562 publications up to April 2020. The inclusion criteria were studies carried out on humans and reporting on the treatment of DH with laser therapy. Case reports, literature reviews and systematic reviews were excluded. Selected by abstract, potentially eligible papers were read in full (n = 160). Independent examiners performed data extraction and the assessment of the risk of bias.
Results. A total of 34 studies were included in the analysis, and 11 in the quantitative analysis. It was observed that most studies followed up patients for a maximum of 6 months (55%). Through the meta-analysis, we observed statistically significant differences between the average pain before and after 3 months of treatment with high- and low-power lasers. However, through indirect comparisons, it was observed that the high-power laser showed a greater tendency to reduce the pain levels after 3 months of treatment as compared to the low-power laser, but without a statistically significant difference.
Conclusions. It was possible to conclude that regardless of the type of laser used in the treatment of DH, this treatment is an effective option for the control of pain symptoms. However, it was not possible to establish a defined treatment protocol, since the evaluation methods are very different from each other.
Text for Rewiew and clinical cases.
Keywords: systematic review, laser, hypersensitivity, therapy, dentin
Introduction
Dentin hypersensitivity (DH) is one of the most commonly encountered clinical problems in dental practice. It is defined as pain resulting from exposed dentin in reply to chemical, thermal, tactile, or osmotic stimuli, which cannot be attributed to any other defect or dental pathology.1, 2, 3, 4 Clinically, it is a localized or generalized sharp pain of quick beginning and can affect one or several dental surfaces.5 DH can have an adverse effect on the quality of life, especially concerning limitations of diet, tooth hygiene, and daily activities.6, 7, 8 A significant association was found between DH and the frequency of tooth brushing in a recent study.9 In addition, poor oral hygiene can result in the emergence of other oral diseases and amplification of DH.9 Hypersensitivity in younger patients is often caused by the exposure of dentin due to erosion, while gingival recession often causes hypersensitivity in older patients due to the exposure of the dentinal tubules in the cervical areas caused by periodontal disease and intensified brushing activity.10
Many theories have been proposed to explain the biological mechanism of DH. For example, dentinal receptor mechanisms, odontoblastic transducer mechanisms, pain receptors on nerves in the pulp, nerves stimulated by hydrodynamic mechanisms, and nerve impulses modulated by the release of polypeptides.11 However, the hydrodynamic system theory is the most accepted, and it suggests that the painful sensation is induced by the stimulation of dentin exposed by the movement of fluid within open dentin tubules in reply to mechanical or osmotic alterations/stimuli. The dislocation of fluid in the interior of the tubules causes intrapulpar pressure, which stimulates the nerve fibers located in the dentin-pulp interface and generates tooth pain.12
There is a range of treatment options for DH, such as toothpaste, potassium-based agents, glutaraldehyde-based agents, varnishes, strontium or acetate chlorides, resins, oxalates, calcium phosphates, and laser therapy.13 All procedures work to obliterate the tubules and/or desensitize pulp receptors,14 but none have produced predictable long-term results. This is thought to be because the physical and chemical processes in the oral environment can result in the tubules being exposed again. The ability to resist the recurrence of pain would be essential for laser therapy to maintain its long-term effectiveness.15
However, many studies have shown laser therapy to be an effective treatment for hypersensitivity.16, 17, 18, 19 The treatment can be accomplished with high or low-intensity laser therapies.16, 18, 19, 20, 21 According to the wavelength of the laser, the action on the dentin surface during the treatment of DH is different depending on the irradiation parameters (mainly wavelength and energy density) and also on the time of treatment.22
The first mechanism of action of the high-power laser occurs by the obliteration of tubules through the partial fusion of dentin substrates.6, 23, 24 The low-power laser decreases the chronic inflammatory process present in the cells, by activating the sodium and potassium pumps and obliterating dentin tubules through the formation of tertiary dentin,25 and can promote analgesic, anti-inflammatory, and biomodulatory effects.17, 22 Laser treatment is well tolerated by patients and is considered safe, quick, and painless.26
A systematic review in 2013 by Lin et al. showed that the different desensitizing treatments used in the office had significantly better results than placebo.27 However, these authors did not stratify the results according to the type of laser evaluated. In the same year, another systematic review showed that both lasers – high and low-power – showed effective results in the treatment of DH.16
In contrast, until now, there are no established treatment protocols in the literature for DH with laser therapy and its long-term effectiveness. Considering these facts, this study aimed to review clinical trials and protocols for DH treatment with laser therapy through a systematic review and meta-analysis, in addition to updating the studies available on the proposed subject.
Methods
This article was written using the PRISMA protocol as a guide for the systematic review available at http://www.prisma-statement.org and was registered in the PROSPERO database (CRD42018095822).
Search strategy and selection criteria
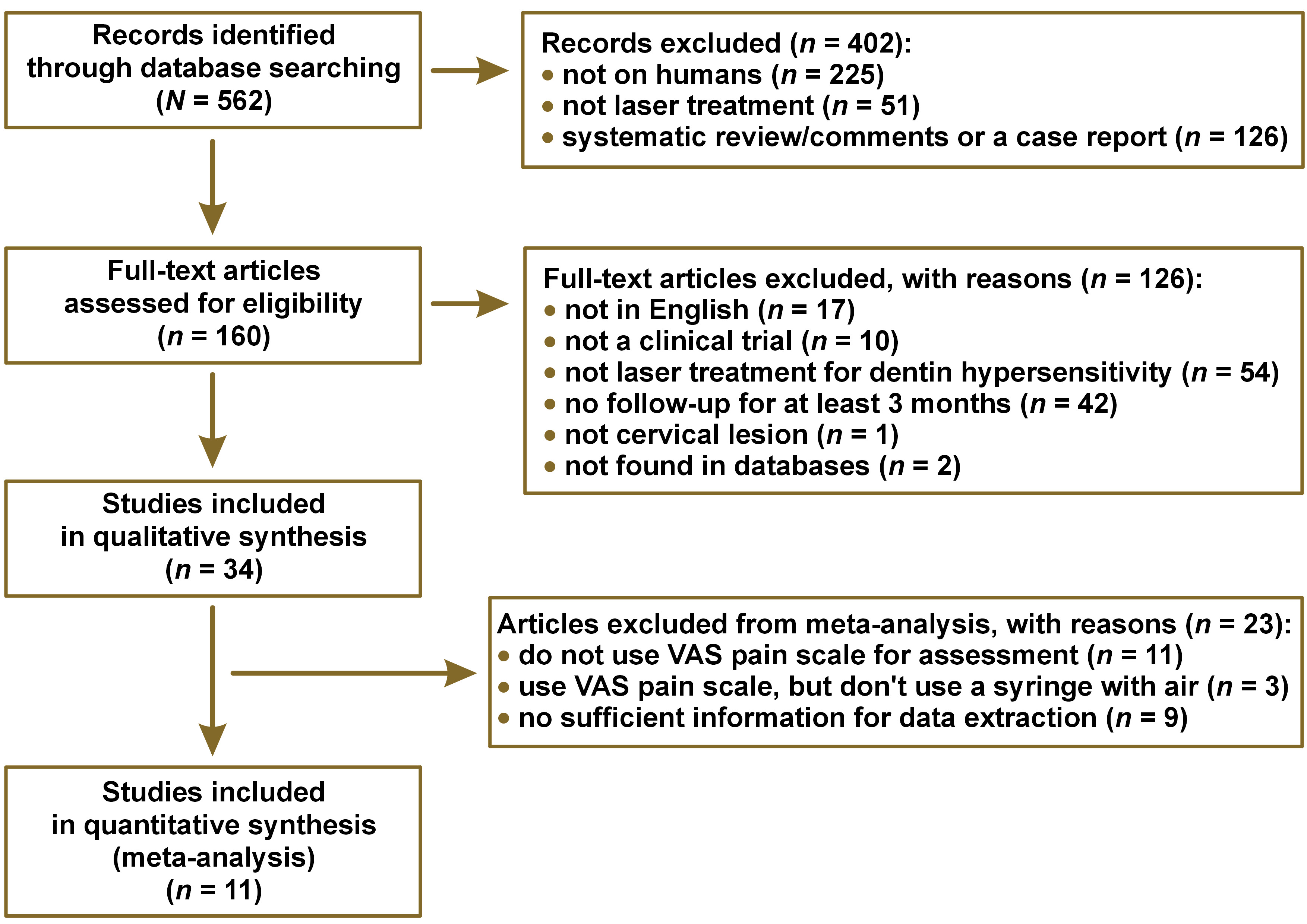
The search was performed and limited to studies in PubMed (MEDLINE) with an English language restriction up to April 2020. The literature search was conducted as displayed in Figure 1.
The selection was conducted by two authors (LAP and LLMM). Each author reviewed the title and abstract of the papers and selected the studies based on inclusion criteria. Disagreements on studies were resolved by a discussion, with the involvement of a third review author when necessary. They were trained to achieve nearly perfect agreement of the Kappa index.
Studies were selected according to the criteria defined below.
The inclusion criteria were: (1) studies carried out on humans; (2) articles that reported on the treatment of DH using laser therapy; and (3) studies that were not case reports, literature reviews, or systematic reviews.
The full text of all eligible articles was subject to the following exclusion criteria: (1) non-randomized clinical trials; (2) not written in the English language; (3) not in permanent teeth; (4) studies without laser therapy treatment; (5) studies that did not have at least 3 months of follow-up after baseline; and (6) studies without sufficient information for data extraction.
Data extraction
The studies that met the inclusion and exclusion criteria were submitted for data extraction. The information was extracted by an author (LAP) and later verified by a second author (LLMM). Both reviewed articles at all stages, and differences were discussed until a consensus was reached.
A standardized data extraction form was used to record the following details:
– study characteristics (author, publication year, title, publication journal, study design, country, setting, funding);
– number of teeth treated;
– participant age;
– study design;
– endpoint assessment of pain;
– follow-up time;
– drop-outs in follow-up;
– mean and standard deviation (M ±SD) values before and after laser therapy treatment – second outcome measures;
– success of intervention judged by pain reduction;
– information about the treatment protocol, such as laser type (high or low-power), application time, number of sessions applied, application range, and laser power and energy – primary outcome measures; and
– adverse events.
If there was not enough information described in the paper, an email was sent to the authors in an attempt to complete the data extraction before it was excluded from the review.
The primary endpoint of interest was to research the protocols existing for DH treatment using laser therapy. The secondary endpoint was to try to verify which DH treatment using laser therapy was more successful through the meta-analysis.
Assessment of the risk of bias
Each selected study was assessed qualitatively using the Cochrane Handbook for the Development of Systematic Intervention Reviews, v. 5.1.0 (Cochrane Handbook). The criteria were divided into main domains associated with randomization, blinding, outcome data, and sample characteristics at baseline. The bias risk assessment was accessed as ‘low risk of bias, ‘high risk of bias, or ‘confuse/unclear’ when it was not possible to identify the information or it was uncertain about the subject or potential bias (classified based on each item of the study criteria). Both reviewers (LAP and JJF) performed independent analyses and disagreement was resolved via a discussion.
Statistical analysis
Direct comparisons were performed between the two treatments using a meta-analysis of random effects. Also, network meta-analyses were conducted to allow indirect comparison between high and low-power lasers. We conducted a Bayesian analysis of mixed treatment comparisons (MTC) to concurrently consider both direct and indirect signs. Studies were performed in similar groups of patients, meeting the assumption of transitivity of this network analysis. First, MTC analyses were computed using both fixed and random models, and the merit of fit for the models was quantified using the residual deviation and the residue information criterion (RIC), with lower scores being better. Because of that, the random effects model with consistent variability among studies was chosen for the final MTC analysis. A node split analysis for unreliability was performed for the pairs with both direct and indirect evidence.
Meta-analyses and network meta-analyses were performed using the R package (GeMTC), v. 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). For each involved study, mean differences (MDs) and 95% confidence intervals (CIs) were estimated.
Results
Study selection
The inter-examiner reproducibility (Kappa index) was 0.85 and considered excellent. The systematic search of the literature identified 562 potentially relevant studies. After removing duplicate studies and screening the titles, abstracts, and/or full texts, 34 studies were included and analyzed qualitatively.
The main reason for not including these studies in the review was that they were not performed on humans (in vivo study, 40%). The remaining 160 studies were analyzed using the full texts for further details and study judgment. Finally, 34 articles matched the eligibility criteria and had adequate data for qualitative analysis, and 11 articles were included in the meta-analysis. The details of the bibliographic research and the selection process of the studies are illustrated in Figure 2.
Study design
The main characteristics of our data sets of longitudinal studies are shown in Table 1.
It was observed that most studies had a split-mouth design (55%), and the age of these subjects ranged from 11 to74 years.
Most of the criteria used for DH assessment and discomfort were reported by the patient. The use of air from a triple syringe followed by the application of a visual probe to scratch the dental surface or perform a thermal test. However, it was also observed that there was no standardization regarding the pain scale used, but most studies use the visual analog scale (VAS) – 70%. The VAS was a 10 cm extension and, with each side, contained an indication of ‘no pain’ and ‘severe pain’.
Follow-up lengths were most frequently 6 months (55%), and only two studies followed the patients for a period of 18 months.
The qualitative analysis included 63 treatment groups, and Table 2 shows the main characteristics of the laser therapy protocols amongst each group, which are described below.
Most of the protocols using lasers with or without another desensitizing agent reduced the pain levels of the patients during the follow-up period. Moreover, the studies that evaluated patients for 18 months had significant results regarding a decrease in pain for all treatments.
The low-power diode laser was the device of choice in 49% of groups that used the low-intensity laser alone or in combination with other therapies. The high-power laser was used alone or combined with other therapies in 55% of the groups that used high-intensity lasers.
It was observed that the average application time for the groups that used a high-power laser was 43 s and that it varied from 1 to 20 applications on the same day. On the other hand, groups using this type of laser did not repeat the treatment protocol on different days, except two studies performed the protocol for three days at intervals of one week per application, and one study performed the protocol on four different days (baseline, 1 week, 1 month, and 6 months). In addition, 31% of the groups evaluated used high-power laser therapy with a desensitizing agent.
In the low-power laser groups, the average application time was 36 s, not exceeding 1 application per day, but being able to repeat applications on different days (maximum 4 applications), except in two groups that performed 4 applications, each lasting 10 s on the same day. Only 9 groups evaluated the use of low-power laser therapy with a desensitizing agent or desensitizing toothpaste.
Risk of bias
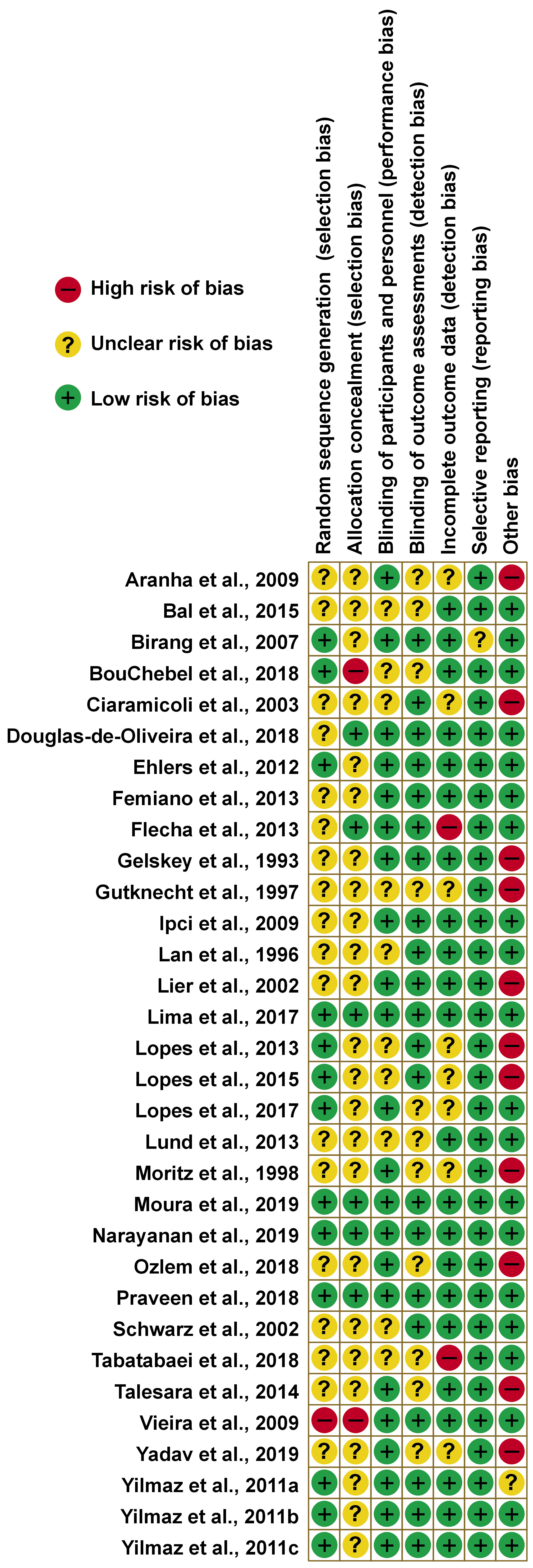
The details of the bias risk assessments for each study are presented in Figure 3. Most studies had one or more “confused/unclear” classifications due to a lack of information. In 40% of the studies, the random sequence generation was described in detail, however, of these, only 4 described the allocation concealment method.
In 68% and 65% of the studies with a low risk of bias had blinding of participants/personnel and outcome assessments, respectively. Regarding incomplete outcome data, the majority had no attrition levels above 20% at the end of the follow-up.
Also, 34% had a high risk of bias concerning the other bias items, as they varied in the number of laser applications administered to the same patient or indicated the use of fluoride-free toothpaste during treatment. In addition, some had a flaw in the methodology that the authors deemed important.
Regarding the articles that were analyzed using the meta-analysis, only 1 did not describe the randomization sequence, 2 did not describe the allocation concealment, and 2 were at high risk due to other biases.
Two studies are not represented in the graph, as they presented only one treatment arm, making it impossible to assess the risk of bias.
Meta-analysis
Only the articles that used the VAS with a syringe of air to assess DH were used for the meta-analysis to standardize the patient’s pain collection. The groups were separated between high and low-intensity lasers using the power described in watts (W) or milliwatts (mW) to perform the meta-analysis separately.
Nine articles were not included in the analysis due to not providing sufficient information about the means and standard deviations before and after treatments. Groups that used desensitizing agents in conjunction with laser therapy were also not included in the meta-analysis.
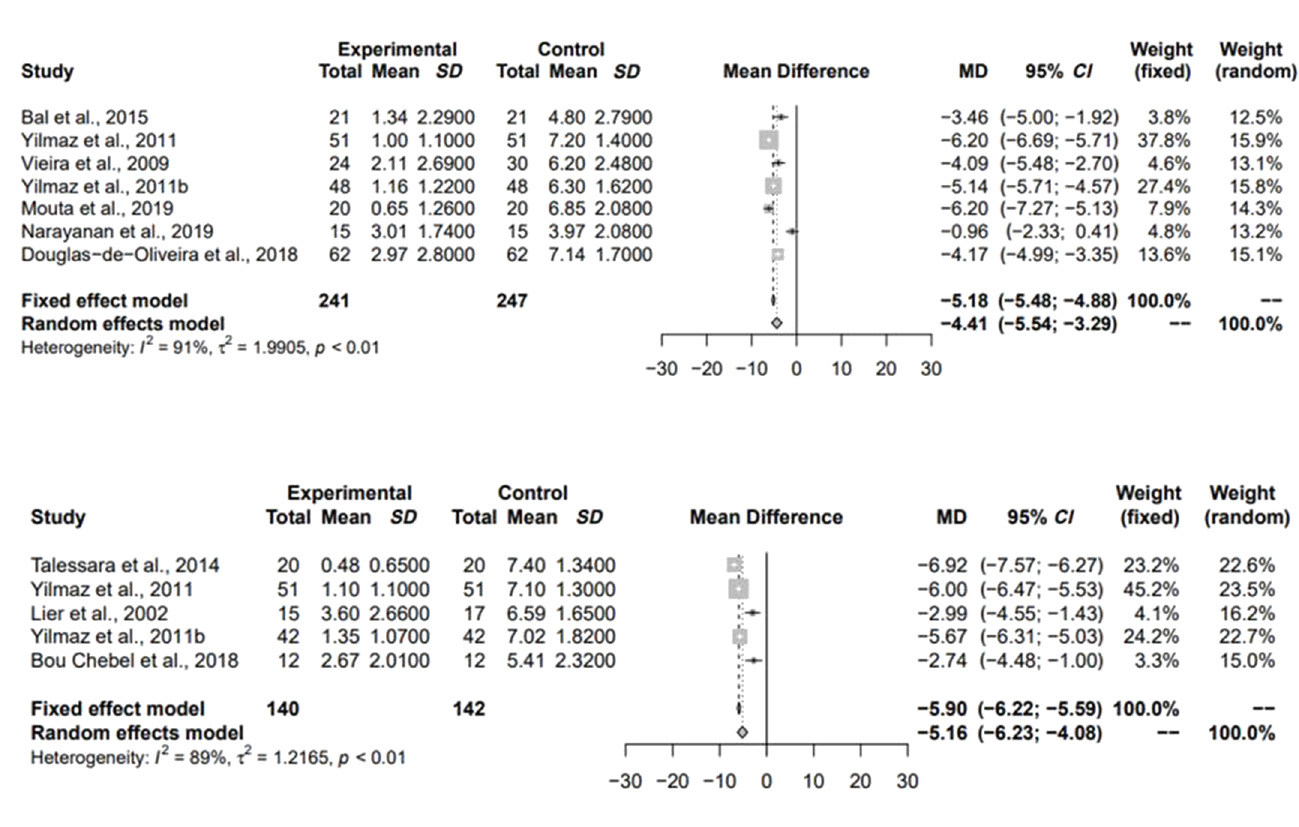
Direct comparisons between the groups
There was a significant difference between average pain levels before and after 3 months of treatment when compared to a treatment control group (before the treatment), regardless of the type of laser used (Figure 4).
Indirect comparisons between the groups
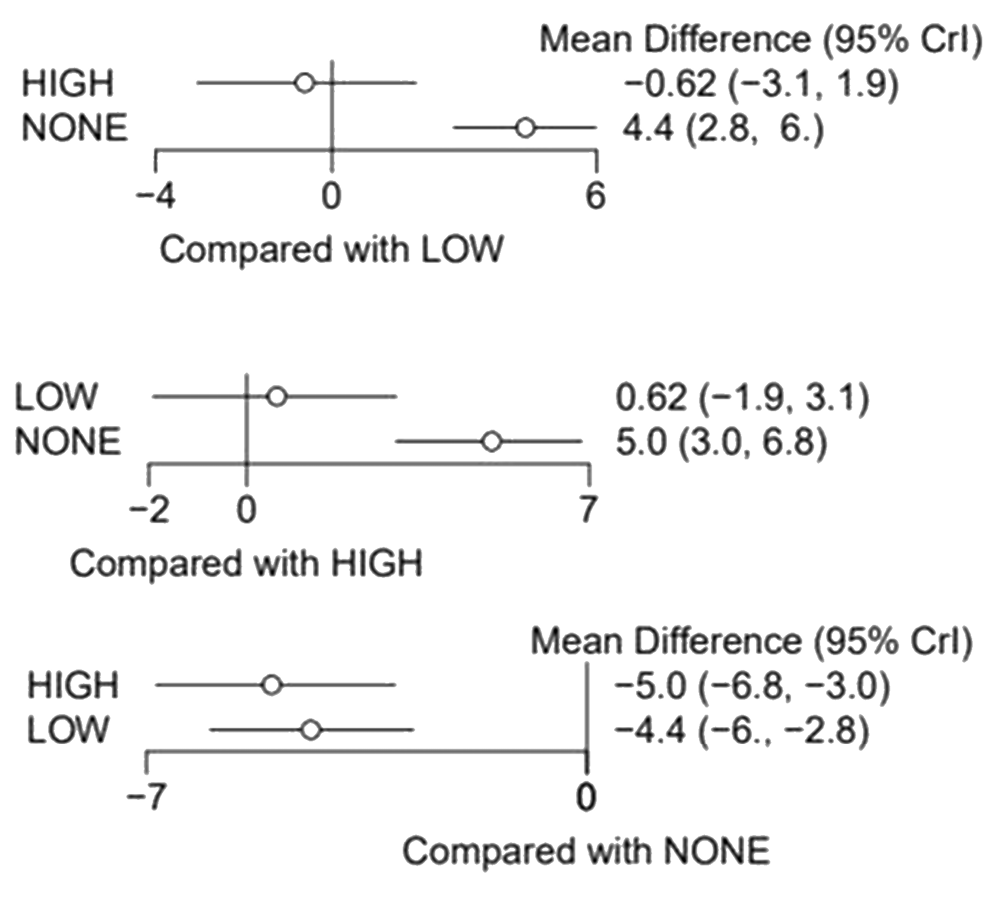
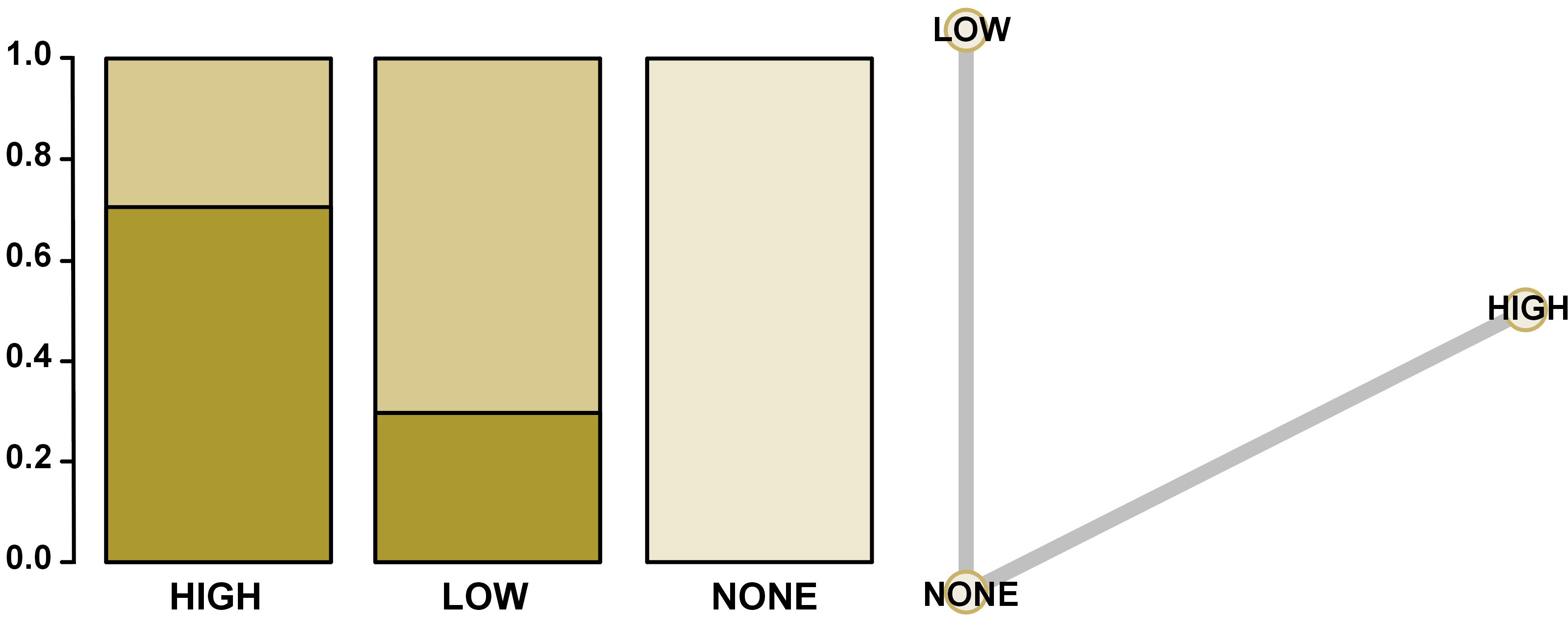
Through indirect comparisons, it was observed that the high-power laser showed a greater tendency to reduce pain levels after 3 months of treatment when compared to the low-power laser, but without a statistically significant difference (Table 3 and Figure 5). Indirect comparisons did not influence the results for low-power versus no treatment and high-power versus no treatment. In that way, there was no inconsistency.
Ranking and network analyses
In Figure 6, it was possible to observe through the ranking and network analysis that the high-power lasers showed a greater chance to be the better treatment option, followed by low-power lasers, and performing no treatment the worst concerning self-reported pain for DH. The network diagram is also shown in Figure 6.
Discussion
Various techniques and different protocols for the treatment of DH have been described in the literature, but no systematic reviews and meta-analyses have been performed to date to evaluate DH treatment protocols. A full search strategy was used, though, to decrease the risk of bias, only randomized controlled trials were chosen for this systematic review.
After including the eligible studies, a qualitative analysis of 34 studies was carried out. In contrast, due to the lack of standardization in pain assessments and the lack of information necessary to perform a quantitative analysis, only 11 studies were considered for the meta-analysis.
Through the meta-analysis, it was possible to verify that the average pain reported by patients was lower after 3 months of follow-up when compared to the average pain before treatment with laser therapy.
The management and treatment of DH are complicated as a consequence of their multifactorial nature, and factors such as parafunctional habits, diet, hygiene, and periodontal and pulp health can influence the results, making research about DH complex and challenging.60 Recently, an improvement in laser technology has allowed laser therapy to emerge as a new treatment alternative for DH.61 There are several methods to assess DH intensity, but the lack of standardization in pain measurements is a difficulty faced by researchers.62, 63 This can be explained by the fact that the perception of each individual’s pain is very subjective, which depends directly on the individual’s ability to define what he is feeling through numerical scales.64 The index most used in the studies and evaluated in the qualitative analysis for pain was the VAS (70%).
Various stimuli can be used, such as tactile stimuli using probes and thermal stimuli using a cold water jet and air spray.65 Differences in the mode of DH assessment can also lead to discrepancies in studies, contributing to the heterogeneity of painful responses. Compared to tactile stimulation, air spray is the most reliable method for assessing DH.66 The meta-analysis of this study included only treatment groups that assessed pain by air-jet using a triple syringe, followed by the application of the VAS in an attempt to standardize some parameters to the maximum.
Other variables, such as the wavelength, power, and energy generated by the laser device, are considered of extreme importance since their variability can directly interfere with their applicability. However, it was also observed that these parameters varied among studies, and most high-power laser studies have not described the wavelength and energy used. Which is different from the groups treated with low-power lasers, which described these main parameters. This can be explained since most high-power lasers have pre-established wavelengths depending on the model of the device, and this value can only vary in low-power models.
Split-mouth studies are preferred in dentistry, as it is possible to minimize inter-individual variables, being the participants are their controls, and when these variables are present, they can interfere in the same way with the treatments. However, in cases where it is necessary to assess the patient’s pain on both sides of the mouth, one of the responses may be influenced by the pain the patient is experiencing on the other side of the mouth. It is important to note that most works in this systematic review were carried out using this form.
The low-power lasers used for the treatment of DH can interact with the dental pulp leading to an increase in odontoblastic activity and, consequently, the production of tertiary dentin and a decrease in the lumen size of dentinal tubules. This type of laser can also stimulate cell activity, promote anti-inflammatory effects, and produce analgesic effects.5, 17, 67 High-power lasers, such as carbon dioxide (CO2) and neodymium-doped yttrium aluminum garnet (Nd:YAG), are the typical types of lasers used in dentistry. These high-power lasers can obliterate the dentinal tubules through fusion and re-solidification by melting the hydroxyapatite and, after cooling, solidifying and forming hydroxyapatite crystals larger than in the initial structure, thus reducing DH.44, 68
Most of the studies evaluated, regardless of the type of laser used, showed satisfactory results regarding DH pain after months of therapy. The low-power laser most used by the authors was the diode laser. It is known that, in addition to having an immediate analgesic effect, it stimulates odontoblasts in the production of irregular dentin that can lead to the obliteration of dentinal tubules,69, 70 and provide analgesia after months of treatment.
Some authors have reported the disadvantages of lasers, including that they are very expensive, intricate to use, and use over time decreases, among others.5 However, new research shows that laser therapy is well tolerated by patients and is safe and effective.26, 38 After performing the meta-analysis of treatments with high and low-power lasers, it was observed that both lasers showed a reduction in DH after 3 months of treatment by alleviating symptoms through the formation of reactionary dentin after irradiation.52, 71
In this work, through indirect comparisons, it was found that the high-power laser had lower average pain scores after 3 months of treatment when compared to the low-power laser or with the average pain before treatments, however, there was no difference between them. But, management for DH with laser therapy in the office constitutes a more expensive and elaborate treatment modality than traditional treatment planning,5 and high-power equipment has a higher cost when compared to low-power equipment. Therefore, as the results of high and low-power lasers are similar, the low lasers seem to be a viable option concerning the cost for the clinician, such as the diode laser. Besides that, no study has evaluated their cost-effectiveness and more long-term studies are needed.
As a limitation of this study, it can be observed that the DH assessment methods are very different among studies, generating heterogeneous responses concerning pain, not only because the instrument of each study is different, but also because pain is a subjective and personal experience. In addition, some studies did not report the complete protocol used for each type of laser, often not being possible to include them in the analyses. Although most studies followed patients for 6 months after treatment (55%), it was decided to perform the meta-analysis with a 3-month follow-up to include more treatment groups in the quantitative analysis.
Regardless of the type of laser used in the treatment of DH, laser therapy is a proven choice for the control of pain symptoms, but it is not possible to establish a defined treatment protocol since the evaluations of each method are very different among the studies in this review. Also, the available signs suggest that high and low-power lasers are effective in decreasing DH after 3 months of treatment.
Conclusions
Laser therapy can be an effective choice for the control of pain symptoms, despite the different lasers and protocols studied for HD. According to the studies, it is suggested that lasers are effective in decreasing DH pain after 3 months of treatment.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets and all supplementary materials generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.