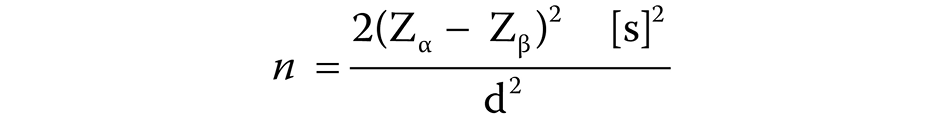Abstract
Background. Biomarkers are emerging, advanced diagnostic tools for the assessment of periodontal disease progression. Omentin-1 is an anti-inflammatory adipocytokine, which has been observed and studied in the saliva of periodontitis patients. Non-surgical periodontal therapy (NSPT) is considered a vital part of periodontal disease treatment.
Objectives. The study aimed to evaluate the interventional effect of NSPT on the levels of salivary omentin-1 in healthy (H) and chronic periodontitis (CP) patients.
Material and methods. A total of 60 participants were selected and equally divided into 2 groups (group A: H participants, group B: CP patients). After obtaining verbal and written consent, whole unstimulated saliva was collected from all participants and analyzed for omentin-1 levels using enzyme-linked immunosorbent assay (ELISA).
Results. Mean salivary omentin-1 levels were elevated and found to be significantly higher in group A (95.80 ±26.65) compared to group B (61.97 ±24.53). In group B, there was a substantial rise in omentin-1 levels from baseline to the 6th week of follow-up (p < 0.001). Thus, NSPT had a positive influence on salivary omentin-1 levels in the treatment group.
Conclusions. Salivary omentin-1 levels may serve as diagnostic and prognostic indicators of periodontal disease progression, and may be used to assess therapeutic outcomes in periodontitis patients.
Keywords: omentin-1, periodontitis, saliva, biomarker, non-surgical periodontal therapy
Introduction
Chronic periodontitis (CP) is a multifactorial, immunoinflammatory disease that affects the vital periodontal tissues supporting the tooth. Although bacterial microflora is the main causative factor in periodontal disease progression, eventually the host’s immune response causes the release of cytokines (e.g., interleukin (IL)-4, IL-10 and IL-35, as well as adipokines, such as omentin-1) that have anti-inflammatory properties controlling further destruction.1 Therapeutic modalities such as scaling and root planing still remain the “gold standard” in non-surgical periodontal therapy (NSPT) and lead to significant improvements in clinical parameters.2, 3
Advancements in oral and periodontal diagnostic research are heading towards more sensitive and objective tools such as biomarkers, which help to overcome the limitations of traditional diagnostic tools.4 A biomarker or biologic marker is a substance that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes or pharmacological responses to a therapeutic intervention.5 Thus, a biomarker for a disease can play vital roles in the diagnosis, monitoring of therapeutic outcomes and drug discovery.6
Researchers concerned with advancements in periodontal disease diagnostics are currently investigating the possible use of oral fluids, such as saliva, for disease assessment. Major salivary gland secretions include proteins and peptides that are responsible for maintaining the integrity of the oral cavity. Salivary components also play a major role in the formation of oral biofilm and host defense; hence, they are associated with the establishment and progression of periodontal disease. A series of initiatives by the National Institute of Dental and Craniofacial Research (NIDCR) has given compelling reasons for the use of saliva as a diagnostic fluid for monitoring periodontal diseases.7 Salivary proteins and RNAs have been used in the detection of oral cancer8 and Sjögren’s syndrome.9 The analyses of the salivary proteomes and transcriptomes are now at the cutting edge of translational and clinical applications in periodontal diseases.7
Although adipocytes (fat cells) occupy a large fraction of salivary gland tissue, little is known about their significance in periodontal disease.10 Many studies11, 12, 13, 14, 15 have reported that adipocytes are able to express cytokines such as adipokines, including salivary leptin, adiponectin and omentin-1.
Salivary omentin-1, a novel anti-inflammatory adipokine, inhibits inflammation via multiple cellular signaling pathways and molecular mechanisms. This adipokine inhibits tumor necrosis factor alpha (TNF-α)-induced cyclooxygenase-2 (COX-2),16 superoxide production17 and the expression of adhesions molecules in endothelial cells, blocking the extracellular regulated protein kinase (ERK)/nuclear factor kappa B (NF-κB) pathway.18 Moreover, omentin-1 plays an anti-inflammatory role in endothelial cells by promoting the AMPK/AKT pathway directly via suppressing the expression of proinflammatory mediators, including TNF-α, IL-6 and monocyte chemotactic protein-1 (MCP-1) in macrophages.19 In addition, omentin-1 promotes the PI3K/AKT signaling pathway that induces the proliferation of human osteoblasts (hOBs).20 Therefore, salivary omentin-1 appears to be a significant regulator of bone remodeling.
In light of the above findings and the paucity of studies evaluating the relationship between periodontal disease and omentin-1, this clinico-biochemical trial aimed to evaluate, compare and correlate the levels of salivary omentin-1 in healthy (H) and CP patients before and after NSPT. The study assessed the validity of omentin-1 as an early diagnostic tool and explored its potential uses for the monitoring of periodontal treatment results.
Material and methods
Preliminary plan and ethics statement
This study was carried out as a prospective, double-blinded, controlled clinical trial. It was approved by the institutional Review Board of the P.M. Nadagouda Memorial Dental College and Hospital, Bagalkot, India, before initiation (approval No. PMNMDCH/1534/2018-19).
The study was carried out in agreement with the ethical standards established by the Declaration of Helsinki. Each patient was given a detailed verbal and written description of the study and a signed consent form was obtained from all of the participants.
Inclusion and exclusion criteria
The patients were assessed at the Ambulatory Care Unit in the Department of Periodontics (P.M. Nadagouda Memorial Dental College, Bagalkot, India), in accordance with the 1999 American Academy of Periodontology guidelines for periodontal disease classification.21 Patients of both sexes, within the age range of 35–60 years and suffering from chronic generalized periodontitis were included in the study. Patients with systemic diseases that could influence periodontal conditions, who underwent periodontal therapy in the past 6 months, who took any systemic medications known to affect the periodontal status, who were pregnant or lactating, who used tobacco in any form, or had a history of radiotherapy were excluded from the study.
Sample size calculation
The required sample size was calculated using the following formula (Equation 1)22:
where:
n – sample size;
Zα – Z variate of alpha error = 1.96;
Zβ – Z variate of beta error = 0.84;
s – standard deviation;
d – difference between the means for the 2 groups.
After the calculation, the estimated smallest sample size was approx. 58. A total of 60 patients were recruited in order to compensate for a 20% dropout of participants who might fail to follow-up.
Periodontal examinations
The oral screening was performed to clinically assess the participants both at baseline and at the 6th week after NSPT. The gingival index (GI),23 oral hygiene index simplified (OHI-S),24 sulcus bleeding index (SBI),25 pocket probing depth (PPD), and clinical attachment level (CAL) were recorded, and unstimulated whole saliva samples (for the evaluation of omentin-1 levels) were collected by a single examiner who was blinded to the patient’s condition.
Collection of unstimulated whole saliva
A total of 1.5 mL of unstimulated whole saliva was collected from each participant. Salivary samples were collected between 9 and 11 am to minimize circadian influences.26 During sample collection, patients were comfortably seated with eyes open, head tilted slightly forward, protected from any stimulation. They made minimal orofacial movements in order for saliva to accumulate in the floor of the mouth. Participants were then instructed to spit into a 1.5-mL Eppendorf tube every 60 s for 10 min or when the participant experienced an urge to swallow the fluid accumulating in the floor of the mouth.27 Samples were centrifuged for 20 min at 1000 g at 2–8°C to remove any particulate matter, sealed firmly and sent to the Maratha Mandal’s Central Research Laboratory (Belgaum, India) where they were stored at −80°C until enzyme-linked immunosorbent assay (ELISA) testing.
Periodontal therapy
After the unstimulated saliva sampling, CP patients underwent thorough subgingival scaling and root planing using ultrasonic scalers (Woodpecker™, Guilin, China). Root planing was carried out using either 4R-4L or 2R-2L Columbia universal curettes (Hu-Friedy, Chicago,
USA) via established techniques. Oral hygiene instructions were given to all individuals, particularly regarding regular tooth brushing, the use of the modified bass technique, and suitable devices for interdental cleaning, such as dental floss and interdental brushes.
Biochemical evaluation
An ELISA kit (ELK Biotechnology Co. Ltd., Wuhan, China) was used to determine omentin-1 levels in the collected saliva and a sandwich enzyme immunoassay was carried out. Samples were added to a microtiter well plate where an enzyme–substrate reaction gave rise to a color change that was measured spectrophotometrically at a wavelength of 450 ±10 nm.
Statistical analysis
The data obtained from the clinical and biochemical evaluations is presented as mean ± standard deviation (M ±SD). The GI, OHI-S, SBI, PD, and CAL data was analyzed using the Student’s t-test. Correlations between the variables in both groups were evaluated using the Pearson’s test. Comparisons of categorical variables were made using the χ2 tests. For all statistical tests, the significance level was set at p < 0.05, keeping α error at 5%, β error at 20%, and giving the study a power of 80%.
Results
A total of 60 participants aged 18–60 years were recruited in the study. No notable differences were observed in the distributions of age and gender (mean age in group A: 38.37 ±8.45 years; and group B: 40.60 ±10.25 years; p = 0.361; gender (p = 0.791)), as depicted in Table 1 and Table 2, respectively.
Clinical parameters
A significant reduction (p ≤ 0.001) was observed in the GI, OHI-S, SBI, PPD, and CAL in the CP patients at 6 weeks after NSPT. Intragroup comparisons between CP patients at baseline and 6 weeks after NSPT showed a statistically significant difference in the mean values for GI, OHI-S, SBI, PPD, and CAL (p = 0.000), as presented in Table 3.
An intergroup comparison of omentin-1 levels showed a statistically significant difference (p = 0.000) in mean salivary omentin-1 levels between healthy participants (95.80 pg/mL) and CP patients (43.10 pg/mL).
Intergroup comparisons between healthy participants and CP patients at baseline showed significant differences in the statistical mean values for GI, OHI-S, Bleeding on probing (BoP), PPD, and CAL (p = 0.000).
Correlation between salivary omentin-1 levels and various parameters
A statistically significant moderate and negative correlation was seen between age and the expression of omentin-1 (r = −0.420, p < 0.05) in CP patients at 6 weeks after NSPT, indicating that as the value of one variable increases, the other reduces (Table 4). However, healthy participants and CP patients showed a statistically non-significant correlation between age and the salivary expression of omentin-1 (r = 0.098, r = −0.315 at baseline, respectively; p > 0.05).
Comparisons of salivary omentin-1 levels between the genders of the participants in both groups showed highly significant differences (p < 0.01), with higher values in healthy group females. Higher values of omentin-1 were noted in males than in females in both groups (i.e., CP patients at baseline and 6 weeks after NSPT); however, the difference was not statistically significant (p > 0.05; Table 5).
Salivary omentin-1 levels when correlated with the clinical parameters (GI, OHI-S, BoP, PPD, CAL) in healthy participants, CP patients at baseline, and CP patients 6 weeks after NSPT, showed statistically non-significant correlations with either a slightly positive or negative relationship (Table 6).
Discussion
The present study was undertaken to evaluate, compare and correlate the levels of salivary omentin-1 with the severity of periodontal disease as assessed by clinical parameters in healthy participants and CP patients at baseline and 6 weeks after NSPT.
The mean age of the healthy participants (38.37 ±8.45 years) was lower than that of CP patients (40.60 ±10.25 years; Table 1); however, this difference was not statistically significant. This finding is in accordance with previous studies conducted by Dogan et al.,1 Balli et al.15 and Bagwe et al.,28 which showed no statistically significant age differences among healthy and CP participants (i.e., indicating an equal distribution as per randomization). Statistically significant moderate and negative correlations were observed between age and the expression of omentin-1 (gingival crevicular fluid (GCF); p < 0.05) in the CP group 6 weeks after NSPT, which suggests that the expression of omentin-1 decreases with age (Table 4). This finding is in line with the study by Bagwe et al.,28 which showed higher levels of omentin-1 in healthy and older age group individuals. Decreased levels of omentin-1 may be supported by the fact that greater numbers of individuals with diabetes or rheumatoid arthritis are in the age groups of 41–59 years29, 30 and 51–69 years,31 respectively, which suggests that the downregulation occurs in aged individuals due to other physiological conditions along with CP.
There was no notable difference in the gender distribution (23 female and 37 male participants; Table 2) in our study, indicating that there was no considerable variation in distribution as per randomization in both groups. Comparisons of omentin-1 expression across gender (Table 5) showed highly significant differences among males and females in the healthy group, with higher omentin-1 levels seen in females. In contrast, higher values were observed in males than females in both the CP at baseline and CP 6 weeks after NSPT groups; however, these values were not statistically significant. To the best of our knowledge, there are no direct studies available in periodontal literature to compare with our results. These findings are in accordance with Luque-Ramírez et al.,32 who studied sexual differences in the circulating levels of adipokines that were caused by the differential effects of sex hormones on adipose tissue. Along with de Souza Batista et al.33 who studied levels of plasma omentin-1 and gene expression in obesity, they found higher plasma omentin-1 levels in lean women than in lean men.
The GI, OHI-S, BoP, PPD, and CAL represent measures of the severity of the inflammatory burden within the gingival tissues and are indicative of periodontal diseases.34, 35 In the present study, we found significantly increased scores in all of these clinical parameters (GI: 2.27 ± 0.45, OHI-S: 4.13 ±0.98, SBI: 3.21 ±0.68, PPD: 3.04 ±1.87 mm, CAL: 3.35 ±1.70 mm). Also, intergroup comparisons showed statistically significant differences in the clinical parameters between healthy participants, CP patients at baseline, and CP patients at 6 weeks after NSPT (Table 3). These results are consistent with a study by Sato et al.,36 which showed higher scores in clinical parameters that could have been the result of the elimination of local etiological factors and reduced inflammation by NSPT.
The present study showed significantly higher levels of salivary omentin-1 in healthy participants (95.80 ±26.65 pg/mL) than in CP patients at baseline (43.10 ±23.47 pg/mL). These findings are similar to the results reported by Bagwe et al.28 who observed increased levels of omentin-1 in GCF and serum. A study by Sarhat et al.37 also showed higher levels of omentin-1 in the serum of healthy participants as compared to CP patients, thereby suggesting an anti-inflammatory activity for omentin-1. Also, previous studies have shown that insulin and inflammation are closely linked38; hence, decreased levels of omentin-1 in CP patients confirm its anti-inflammatory role.
Correlations between salivary omentin-1 levels and clinical parameters in healthy participants (Table 6) showed a slightly positive and negligible relationship with GI, and a slightly negative and negligible relationship with OHI-S and BoP. The values were not statistically significant (p > 0.05). Correlations of salivary omentin-1 levels with clinical parameters in CP participants at baseline showed a slightly positive and negligible relationship with GI and BoP, and a slightly negative and negligible relationship with OHI-S, PPD and CAL. These results were also statistically non-significant. The results for both healthy participants and CP patients at baseline were in contrast to the studies by Dogan et al.1 and Balli et al.,15 which showed statistically significant and negative correlations with CAL and GI, suggesting an improvement in the periodontal health condition. Correlations of omentin-1 levels with clinical parameters in CP patients at 6 weeks after NSPT showed a slightly positive and negligible relationship with BoP and PPD, and slightly negative and negligible relationships with GI, OHI-S and CAL. The values were not statistically significant. The weak correlations between the levels of omentin-1 and clinical parameters suggest that a larger sample size is required for a more precise and accurate assessment of the relationship between both variables.
Limitations
This research involved certain limitations, such as a smaller sample size and short duration of the study. The smaller sample size and various other differences, such as race and population variations, do not allow these findings to be generalized to the entire population. Therefore, further long-term clinical investigations using a larger sample size, multiple centers and various races are required to confirm our findings.
Future perspectives
The results of our study suggest that salivary omentin-1 levels can serve as reliable biomarkers for the diagnosis and prognosis of periodontal disease, as well as the assessment of treatment outcomes. The levels of omentin-1 decline in various pathological conditions. Thus, appropriate steps taken to maintain its adequate levels, such as weight loss,39 an olive oil-rich diet40 or aerobic training,41 may guarantee a long and healthy life. Treatment with atorvastatin and certain anti-diabetic drugs, such as metformin or pioglitazone, is also effective at increasing endogenous omentin-1 levels by improving insulin sensitivity.
These results may contribute to the development of omentin-1-based medicines, such as omentin-1 analogs and omentin-1 receptor agonists, to combat various inflammatory conditions, including periodontitis and metabolic disorders.
Conclusions
The present research evaluated the effects of NSPT on the levels of salivary omentin-1 in periodontally healthy participants and CP patients. Within the constraints of this study, it can be stated that lower salivary omentin-1 levels were identified in CP patients. They were associated with increased levels of periodontal parameters. Higher salivary omentin-1 levels after NSPT were also concomitant with an improvement in the patient’s periodontal health status. Taken together, it may be concluded that omentin-1 has an anti-inflammatory role in periodontitis, and its expression may have a potential role in the immunopathogenesis of CP. Hence, omentin-1 can serve as a biomarker for the early detection and diagnosis of periodontitis, which helps in meticulous treatment planning and the development of proper therapeutic modalities that not only enhance the maintenance of periodontal health, but also improve overall systemic well-being.
Ethics approval and consent to participate
The present study was approved by the institutional Review Board of the P.M. Nadagouda Memorial Dental College and Hospital, Bagalkot, India (approval No. PMNMDCH/1534/2018-19). Written informed consent was obtained from all participants.
Data availability
All data generated and/or analyzed during this study is included in this published article.
Consent for publication
Not applicable.