Abstract
Background. Despite the fact that sleep bruxism (SB) is common in migraine, and that patients with migraine often report poor sleep quality, SB and sleep architecture in chronic migraine (CM) have not been fully explored.
Objectives. The aim of the study was to establish the association between SB and CM, with an assessment of sleep structure alterations in CM.
Material and methods. The diagnosis of migraine was made using the 3rd edition of the International Classification of Headache Disorders (ICHD-3). Sleep bruxism and sleep structure were assessed using polysomnography, according to the American Academy of Sleep Medicine (AASM) Guidelines. All results were adjusted for medication use in the treatment of migraine, which may interfere with sleep and SB.
Results. A total of 110 patients with migraine (mean age: 39.3 years; 88% female) were evaluated, including 65 individuals with CM and 45 episodic migraine (EM) patients. The patients with CM had lower REM sleep duration when compared to those with EM (median (Me): 21.4% of total sleep time (TST) vs. 24.4% of TST, p = 0.008), while REM sleep below 23.1% of TST was associated with increased odds of CM (odds ratio (OR): 3.61 (95% confidence interval (CI): 1.60; 8.15), p = 0.002). Seventy-six out of 110 (69%) participants were diagnosed with SB. The presence of mixed bruxism at a frequency of above 0.4 episodes per hour (n/h) was associated with increased odds of CM (OR: 2.40 (95% CI: 1.06; 5.46), p = 0.048). However, severe SB (bruxism episode index (BEI) >4) was associated with increased odds of migraine with aura (MwA) (OR: 2.68 (95% CI: 1.05; 6.83), p = 0.044). Migraine without aura showed a weak, negative correlation with BEI (r = −0.293, p = 0.002).
Conclusions. A decrease in the REM stage of sleep was associated with CM. Despite the high prevalence of SB in patients with migraine, SB was not associated with CM, while severe bruxism was associated with MwA. Therefore, if any association between SB and migraine exists, it is more likely related to aura phenomena than to migraine chronification.
Keywords: sleep, pain, bruxism, headache, chronification
Introduction
Primary headaches are characterized by the absence of underlying causes, such as injury, disease or pathological processes. These headaches are further classified into migraine, tension-type and cluster headaches.1, 2 Migraine is the third most disabling neurological disorder and affects 15.2% of the general population, mainly young females.3 Due to the frequency of attacks, migraine can be classified as episodic (EM) or chronic (CM). In CM, headache attacks are present at least 15 days a month, with a minimum of 8 days of migraine.4, 5 Chronic migraine is considered more severe and is associated with increased disability, depressive symptoms and the presence of sleep disturbances.4, 6, 7, 8, 9, 10
Sleep bruxism (SB) is defined as rhythmic (phasic) or non-rhythmic (tonic) masticatory muscle activity during sleep.11 Sleep bruxism affects 22% of the global population, and its clinical signs include oral mucosa damage, tooth wear, masticatory muscle pain, or headache.12, 13, 14 Even though SB is not considered a movement disorder,11 it is associated with oxidative stress, decreased quality of life and sleep alteration.15, 16, 17
The exploration of specific sleep disorders and sleep structure in patients with migraine is a subject of research interest. The literature presents contrasting results concerning SB in migraine patients. Several studies have confirmed the relationship between SB and migraine,18, 19 but, on the other hand, there is a lack of association between SB diagnosis and migraine in studies based on video-polysomnography (vPSG), which is the gold standard for SB diagnosis.15, 20, 21, 22 Additionally, there is an absence of data regarding the association between SB and CM, as measured using objective methods. Only 1 study has demonstrated a close association between SB and CM.19 However, it should be noted that SB was diagnosed in this study using questionnaires.19 The association between SB and migraine is poorly explained, despite the increased prevalence of SB in individuals with migraine.19, 22 Moreover, patients with migraine often report reduced sleep quality. However, objective methods used to measure sleep architecture do not corroborate these complaints.23, 24 Yet, these results were calculated in comparison to patients without headaches; therefore, there is a paucity of studies assessing sleep structure in particular forms of migraine such as CM using vPSG.
In order to fill the gap concerning associations between SB, sleep structure and migraine forms, the main objective of the present study was to evaluate the associations between CM and SB using objective methods such as PSG and validated criteria for headache diagnosis, namely the 3rd edition of the International Classification of Headache Disorders (ICHD-3). The second aim was to assess sleep structure in CM patients in comparison to EM participants. We theorized that patients with CM may exhibit alterations in their sleep structure and diminished sleep efficacy. Additionally, we hypothesized that CM participants may have a greater number of SB episodes in comparison to patients with EM, and that SB is positively associated with CM.
Material and methods
The present study was approved by the Bioethics Committee of Wroclaw Medical University, Poland (approval No. KB/25/2024). The study was performed according to the principles outlined in the Declaration of Helsinki for experiments involving human participants.
Study participants
The participants of the study were patients at the Headache Center in the Department of Neurology at Wroclaw Medical University, Poland. The diagnosis of migraine was made based on the ICHD-3 criteria.5 In order to exclude other potential causes of secondary headache, all patients underwent brain magnetic resonance imaging (MRI). Patients with CM received preventive treatment comprising topiramate, onabotulinumtoxinA and monoclonal antibodies (mAbs) targeting the calcitonin gene-related peptide (CGRP) pathway. None of the participants were undergoing active SB treatment in the form of pharmacotherapy, dental appliances or botulinum injections. The treatment of EM encompassed acute treatment in the form of non-steroidal anti-inflammatory drugs (NSAIDs) and triptans. The prophylactic treatment regimen was based on venlafaxine, amitriptyline, propranolol, and antihypertensive drugs. The aforementioned pharmaceuticals were administered in minimal doses when compared to standard therapeutic doses.25 Interviews were conducted with the patients from the Headache Center, with a focus on their sleep-related concerns. Reports of any sleep complaints allowed the referral of patients to the Sleep Laboratory at the Department of Diabetology, Hypertension, and Internal Diseases at Wroclaw Medical University for the diagnosis of sleep disorders.
Sleep architecture and sleep bruxism diagnosis
In the Sleep Laboratory, a single-night vPSG was performed on patients with suspected sleep disturbances. Sleep bruxism was diagnosed using vPSG, which is the gold standard for SB diagnosis.26 Based on electromyography (EMG) recordings as well as audio and video recordings of the bilateral masseter, the following parameters were analyzed: the bruxism episode index (BEI); phasic bruxism, characterized by more than 2 cyclic phasic EMG increases lasting 0.25–2.00 s; tonic bruxism, characterized by episodes lasting >2 s; and mixed bruxism, which is a combination of the aforementioned types. The scoring of all SB episodes occurred under specific criteria, namely, when the activity exceeded at least twice the background EMG amplitude and remained above 3 s of stable EMG. Sleep bruxism was classified as mild (BEI: 2–4), severe (BEI > 4) or absent (BEI < 2). The vPSG device utilized was Nox-A1 (NOX Medical, Reykjavík, Iceland), which consisted of electroencephalography (EEG), electrooculography (EOG) and electrocardiography recordings. The pulse and oxygen saturation levels of the subjects were assessed using a pulse oximeter (WristOx2® 3150; Nonin Medical Inc., Plymouth, USA). The American Academy of Sleep Medicine (AASM) standard criteria for sleep scoring were used by an experienced physician to analyze the vPSG recordings in 30-s epochs.27 The evaluated sleep parameters were as follows: total sleep time (TST); sleep efficiency (SE); sleep latency (SL); wake after sleep onset (WASO); sleep stages, including non-rapid eye movement (NREM) (N1, N2, N3) and rapid eye movement (REM); percentage of TST spent in N1, N2, N3, and REM stages of sleep; apnea–hypopnea index (AHI); and periodic limb movement in sleep (PLMS).
Questionnaires
The participants completed questionnaires that measured their headache-related disability, anxiety, level of daytime sleepiness, level of stress, and habits (drinking coffee, tea or alcohol). Headache-related disability was measured using the Migraine Disability Assessment (MIDAS) questionnaire. The general level of daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS). The severity of anxiety was measured using the Generalized Anxiety Disorder Assessment (GAD-7). The Perceived Stress Scale (PSS) was used to evaluate the extent of stress experienced by the participants in their daily lives. Current alcohol consumption was confirmed if individuals diagnosed with migraine consumed any type of alcohol more than once per day. Current coffee and tea consumption was documented in patients with migraine who reported having at least 1 cup of tea or coffee during a 24-h period.
Eligibility criteria
The inclusion criteria for the present study were adult participants with a definitive migraine diagnosis and with technically appropriate single-night vPSG results who signed informed consent to participate in the study. The exclusion criteria were as follows: age <18 years; coexisting primary or secondary headaches; significant psychiatric, autoimmune and systemic diseases; migraine attack during the vPSG examination; lack of informed consent; absence of completed questionnaires describing stress, anxiety and headache-related disability; malignancies; pregnancy and lactation.
Statistical analysis
The STATISTICA software, v. 13.3 (TIBCO Software Inc., Palo Alto, USA), was used for the calculations. The compliance of the empirical distributions of continuous variables with the theoretical normal distribution was verified using the Shapiro–Wilk test. The homogeneity of variance of the results was checked using the Bartlett’s test. For quantitative variables, medians (Me), lower quartiles (Q1) and upper quartiles (Q3) were calculated. The significance of differences in mean values (medians) of continuous variables with a non-normal distribution or with non-homogeneous variances in 2 independent groups was verified using the Mann–Whitney U test. Nominal and ordinal qualitative variables were presented in tables as numbers (n) and percentages (%). For categorical data, the Pearson’s χ2 test of independence or Fisher’s exact test were used. Spearman’s rank correlation coefficients (rho) or point biserial correlation (rpb) were used to assess the direction and significance of the relationship between 2 variables. To assess the influence of medications that interfere with sleep such as amitriptyline, venlafaxine and topiramate, used in the treatment of patients with migraine, a non-parametric analysis of covariance using the Quade test was performed. This analysis was conducted with the use of the R software with the npsm package (R Foundation for Statistical Computing, Vienna, Austria). For binary variables, Mantel–Haenszel adjusted odds ratios (ORs) were estimated to remove the influence of the confounding variable (“taking medications interfering with sleep”). All statistical hypotheses were verified using two-sided tests at a significance level of p < 0.05. Bonferroni correction was implemented in the data analysis. Prior to the study, no statistical power calculation was conducted.
Results
The study included 110 Caucasian patients with migraine: 97 (88%) females and 13 males, with a mean age of 39.3 years. There were 65 CM and 45 EM patients. The mean age of EM patients was 39.5 years and of CM patients – 39.1 years. The study population included 46 individuals with migraine with aura (MwA) and 64 migraine without aura (MwoA) patients. The patients with CM did not differ in terms of age, sex, body mass index (BMI), anxiety, stress, daytime sleepiness, current alcohol, tea or coffee consumption, medication intake such as amitriptyline, venlafaxine, topiramate, propranolol, or mAbs, and MwA occurrence in comparison to patients with EM (p > 0.05). The headache-related disability level, as measured by the MIDAS score, was increased in patients with CM (Me: 60.0 points vs. 24.0 points (EM patients), p < 0.001). The characteristics of the study sample are collated in Table 1.
In terms of sleep structure among patients with migraine, a decline in the REM stage of sleep was observed in CM patients compared to individuals with EM (Me: 21.4% of TST vs. 24.4% of TST, respectively; p = 0.008). Other sleep parameters did not differ between the groups (p > 0.05) (Table 2).
Seventy-six out of 110 (69%) study participants were diagnosed with SB. There were 38 patients with mild SB (BEI > 2) and 38 patients with severe SB (BEI > 4). However, the median BEI was not increased in the CM group in comparison to the EM group (Me: 2.8 n/h vs. 2.6 n/h, respectively; p = 0.400). Additionally, the phasic, tonic and mixed SB episodes, as well as the duration of all SB episodes did not differ between the groups (p > 0.05). The characteristics of SB are presented in Table 3.
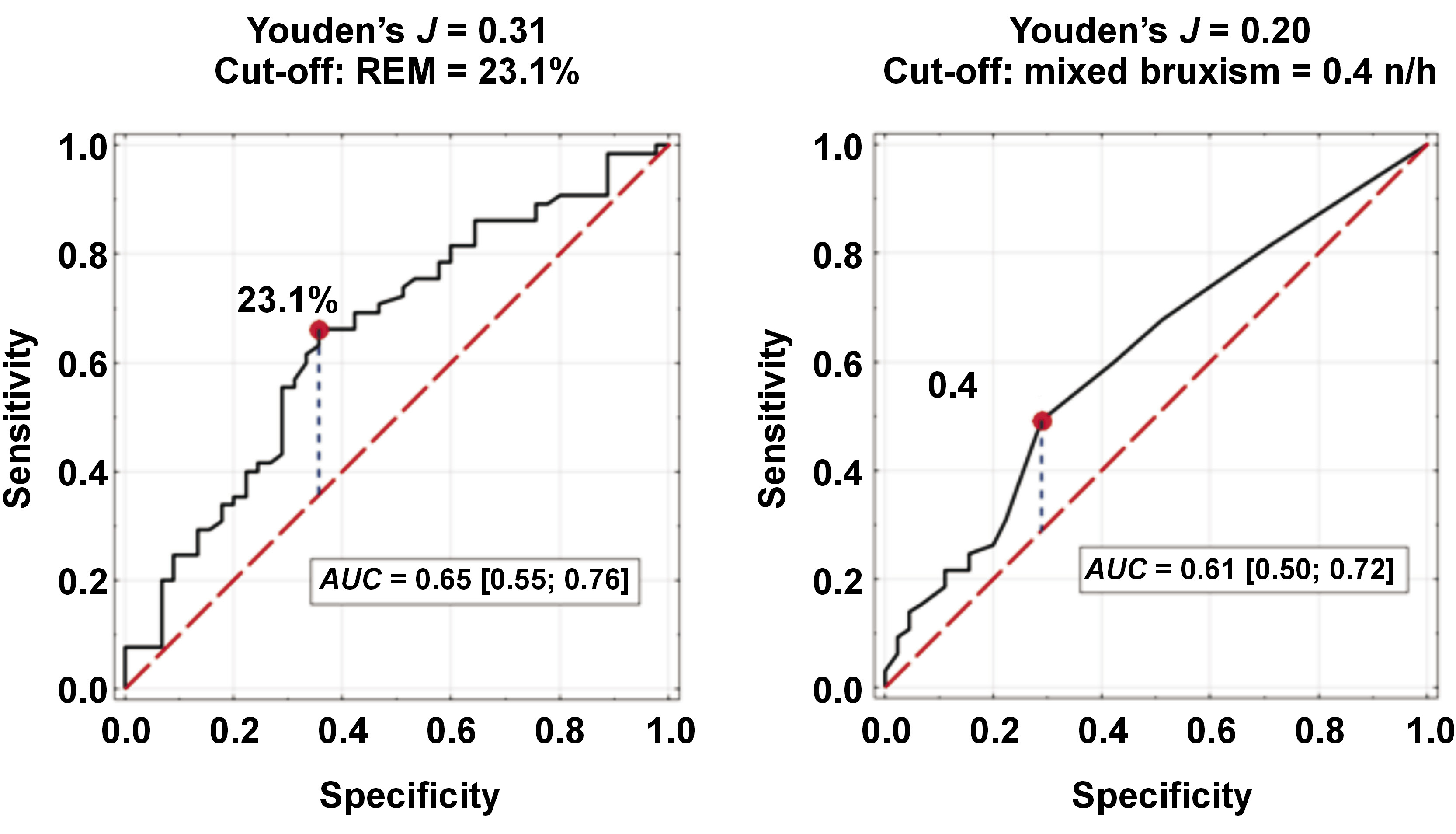
Factors associated with an increased likelihood of CM included REM sleep ≤23.1% of TST and mixed bruxism episodes ≥0.4 n/h (aOR: 3.61 (95% confidence interval (CI): 1.60; 8.15), p = 0.002; aOR: 2.40 (95% CI: 1.06; 5.46), p = 0.048, respectively). Cut-off values for REM sleep and mixed SB episodes were determined based on the analysis of receiver operating characteristic (ROC) curves (Figure 1). Sleep bruxism (BEI > 2) was not associated with CM in comparison to EM (aOR: 0.73 (95% CI: 0.32; 1.65), p = 0.290) (Table 4).
Table 5 presents a comparison of population characteristics and sleep architecture between migraine patients with severe SB and those with mild SB. Increased age and female sex were more frequent in migraine patients with mild SB in comparison to severe SB (mean (M): 40.5 years vs. 35.5 years, adjusted p = 0.049; 94.7% of females vs. 78.9% of females, adjusted p = 0.048, respectively). As illustrated in Table 6, severe SB (BEI > 4) was associated with MwA in comparison to MwoA (OR: 2.68 (95% CI: 1.05; 6.83); adjusted p = 0.044).
Table 7 presents the values for Spearman’s rank correlation coefficient (rho) and point biserial correlation coefficient (rpb) between BEI (n/h) and female sex, CM, MwoA, MIDAS score, and age. Migraine without aura, as well as female sex and age demonstrated a weak negative correlation with BEI (r = −0.293, p = 0.002; r = −0.246, p = 0.010; and r = −0.268, p = 0.005, respectively). The MIDAS score and CM diagnosis were not correlated with BEI (r = 0.071, p = 0.458; and r = −0.012, p = 0.904, respectively).
Discussion
Sleep structure in chronic migraine
Sleep structure is altered in patients with CM in comparison to those with EM. A reduction in REM sleep was noted in these patients. Therefore, the null hypothesis was partially confirmed, because apart from REM sleep, no significant alterations were observed in other sleep parameters. It has been established that REM sleep is decreased in patients with migraine.23 However, a recent meta-analysis suggests that other sleep parameters remain unaltered in individuals with migraine.23 This is an interesting fact given that patients with migraine often report sleep disturbances, which are not confirmed by vPSG.23, 24 These results were made based on the comparison of migraine patients with healthy controls. However, there are rare reports comparing sleep structure based on migraine subtypes using vPSG. Research has demonstrated that CM reduces NREM3 duration and sleep efficiency in comparison to chronic tension-type headache (TTH).28 Orzeszek et al. suggested that the severity of orofacial pain and headaches is not associated with alterations in sleep quality.29 In the present study, the CM group demonstrated a decrease in REM sleep, the role of which is memory consolidation, dreaming, thermoregulation, and emotional processing.30 Previous studies have proven that migraine is associated with dysfunction of memory processing.31 Therefore, it is possible that patients with migraine have impaired memory processing resulting from the decreased amount of REM sleep. However, it must be reiterated that these results are calculated in comparison to the control group, which does not include individuals with migraine. The findings of this study indicate that CM patients may exhibit impaired memory processing compared to patients with EM due to reduced duration of REM sleep. However, there is a paucity of literature focusing on this topic, and further research must be conducted to explain this theory. At the same time, the diagnosis of SB is associated with alterations in sleep structure.32 Severe SB (BEI > 4) has been shown to decrease NREM3 sleep while increasing NREM1 and REM sleep.33 However, in our study, there were no differences in sleep structure following the division of patients with migraine based on SB severity. Thus, it appears that SB does not alter sleep in patients with migraine. Migraine treatment may also have an influence on sleep structure, because it consists of the administration of amitriptyline, venlafaxine and topiramate, which can affect sleep.34, 35 However, this confounding factor was considered in the analysis and, after adjustment, the results still showed that REM sleep was decreased in patients with CM. Therefore, the observed reduction in REM sleep may be unique to migraine chronification.
Sleep bruxism in migraine
A high prevalence of SB was observed in migraine, with only mixed SB episodes above 0.4 n/h demonstrating a correlation with CM. Similar results were observed in a study that explored the associations between SB and migraine in a temporomandibular disorder (TMD) group using vPSG.22 Particular types of SB episodes are still subjects of research, but it has been shown that tonic SB episodes are associated with sleep-related breathing disorders.36 Therefore, it is necessary to explore this topic in further studies and establish whether mixed episodes are one of the hallmarks of migraine or are concomitant findings among patients with migraine. Despite the fact that the present study was focused on the associations between SB and CM, it was discovered that severe SB was related to MwA. Additionally, a weak and negative correlation was identified between BEI and MwoA. Aura is described as a transient neurological symptom that occurs from 5 to 60 min prior to the onset of a headache attack. The most common form of aura are visual disturbances.37 The pathopsychological theory of aura embraces cortical spreading depression and changes in brain vasculature.38 Previous research has indicated that SB may be centrally regulated from the brainstem.39 Contemporary understanding suggests that the etiology of SB is multifactorial.40 These results imply the potential of shared etiology between aura and SB. However, further studies are needed in this regard.
Association between chronic migraine and sleep bruxism
Contrary to the null hypothesis of this study, no association was found between overall SB and CM. However, previous research has demonstrated a positive correlation between CM and SB, with the co-occurrence of TMD in SB further increasing this association.19 The diagnosis of SB in the mentioned study was based on questionnaires, and these associations were calculated in comparison to a control group without headaches.19 This difference may also stem from the fact that, in our study, SB was diagnosed using vPSG, and our primary objective was not focused on identifying and including TMD conditions. Additionally, no correlation was observed between BEI values, which are used in SB diagnosis, and MIDAS scores. However, MIDAS scores did not differ between patients presenting with migraine and severe SB and those with mild SB. Generally, patients with CM have significantly increased life disability due to headaches, with this severity being associated with the MIDAS score.41 Therefore, the lack of association between BEI and MIDAS may indicate that the overall SB diagnosis is not associated with migraine. However, Memmedova et al. demonstrated that patients with bruxism and combined headaches, including migraine, TTH and trigeminal autonomic cephalalgia (TAC) exhibited increased MIDAS scores in comparison to those without bruxism.42 However, the mentioned study was not based on vPSG, and the study group included more than one type of headache. Therefore, taking into consideration all the presented aspects, SB may be related to aura phenomena in migraine rather than migraine chronification.
Limitations
Despite the use of vPSG and the ICHD-3 criteria in the present study, some limitations must be considered. First, the sample size was constrained due to the limited accessibility of vPSG. Prior to the study, a statistical power calculation was not performed. Additionally, the questionnaires may not adequately capture the level of stress, headache-related disability and anxiety in patients with migraine. The temporal relationships between SB and CM were not examined. Patients with migraine received treatment in various forms, and these may have influenced the observed results. In SB like in migraine, botulinum is considered an effective treatment; however, doses of other toxins and other regions of the face muscles are included in the treatment of SB.43 Additionally, the first-line SB therapy is pharmacotherapy, dental appliances or behavioral methods rather than botulinum injections44; therefore, the influence of botulinum on SB results may be non-relevant. Video-polysomnography was conducted without an adaptive night, and the influence of the first-night effect on vPSG results may be significant. Therefore, the presented observations must be interpreted with caution.
Conclusions
A decrease in REM sleep was associated with CM. Despite the high prevalence of SB in patients with migraine, SB was not associated with CM, while severe SB was associated with MwA. Therefore, if any association between SB and migraine exists, it is more related to aura phenomena than to migraine chronification. Further studies are necessary to elucidate these results and to better understand the relationship between sleep disturbances in CM.
Ethics approval and consent to participate
The study was approved by the Bioethics Committee at Wroclaw Medical University, Poland (approval No. KB/25/2024).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.