Abstract
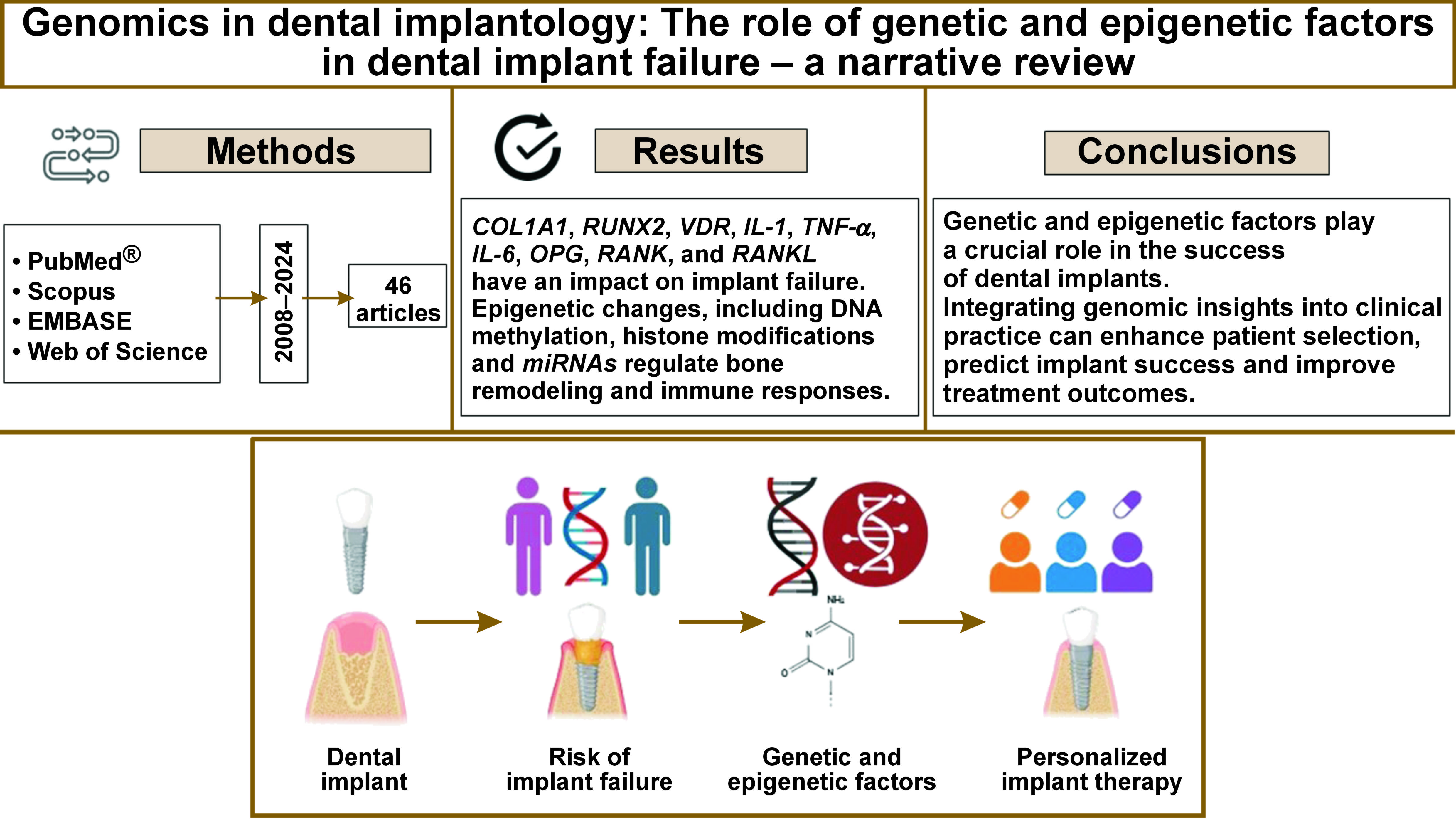
Dental implants are a widely used solution for tooth replacement, yet implant failures remain a challenge. Genetic predispositions and epigenetic modifications influence osseointegration and peri-implant health. The present review explores genetic mechanisms affecting implant healing and introduces implantogenomics – a personalized approach to implant therapy based on an individual’s genetic profile.
A comprehensive review of literature from PubMed®, Scopus, EMBASE, and Web of Science (2008–2024) was conducted using Medical Subject Headings (MeSH) terms such as “genetic markers,” “implantogenomics” and “epigenetics.” After removing duplicates and screening for relevance, a total of 46 studies were included in the analysis.
Key genetic variants in bone metabolism (collagen type 1 alpha 1 (COL1A1), runt-related transcription factor 2 (RUNX2), vitamin D receptor (VDR)), immune response (interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), IL-6), and osseointegration-related genes (osteoprotegerin (OPG), receptor activator of nuclear factor kappa B (RANK), receptor activator of nuclear factor kappa-B ligand (RANKL)) were identified as potential contributors to implant failure. Epigenetic modifications, including DNA methylation, histone changes and microRNAs (miRNAs), regulate bone remodeling and immune responses, and have an influence on implant integration.
Advances in genomics have paved the way for personalized implant therapy through genetic screening, optimizing outcomes and reducing the number of implant failures. Implantogenomics is aimed at tailoring treatments based on genetic profiles, while epigenetic therapies, such as gene modulation, enhance implant integration. Future research should focus on predictive biomarkers and precision-based strategies to improve implant longevity.
Genetic and epigenetic factors play a crucial role in the success of dental implants. Integrating genomic insights into clinical practice can enhance patient selection, predict implant success and improve treatment outcomes. Further research is necessary to establish predictive biomarkers and targeted interventions.
Keywords: genetic factors, epigenetics, implant failure, dental implant, genetic markers
Introduction
Over the past 30 years, dental implants have evolved and become the preferred treatment option for tooth replacement by dentists and patients. The survival rate of implant-supported restorations has increased from 94.6% to 97.1% over the past 2 decades.1 Despite this high success rate, there has been a predominant increase in the incidence of dental implant failure. Previous studies have noted a dental implant failure rate ranging from 1% to 19%.2 Early implant failures have been attributed to altered wound healing, preventing osseointegration, while late implant failures have been associated with extensive peri-implant bone loss after functional loading.3 Understanding the cause of implant failures is essential for their prevention.
Knowledge regarding the factors influencing implant failure and meticulous observation of the implant after placement are crucial elements in the management of the complications such as inflammation, proliferation and progressive bone loss in and around the implant, compromised aesthetics, prosthesis failure, soft tissue dehiscence, implant fracture, and ultimately, implant failure.4 Among all variables contributing to dental implant failure, the host factor has emerged as a contentious risk component.2 Many researchers have sought to uncover the association between alleles and/or genotypes of genetic markers and the predisposition to implant failure. Analyzing these genetic elements associated with dental implant loss may offer insights into the factors contributing to the varied patient response to currently available treatment options.5
Clinicians are able to assess the risk of complications in patients with a negative host response before any elective surgical procedure, as such a response may lead to implant rejection, wherein the host body fails to integrate with the implant. Thus, it is evident that the incidence of implant failure is higher in a subset of individuals who demonstrate a definitive host characteristic, such as genetic factors that disturb the process of osseointegration.6 This phenomenon of a small number of patients concentrating risk for implant loss has been termed “clusterization”.7 High-throughput methodologies are increasingly employed to gain a comprehensive understanding of cellular processes, enabling faster discoveries in health and disease research.8
The present review aims to summarize the genetic mechanisms underlying osseointegration healing and introduce implantogenomics, a concept that applies personalized medicine to tailor implant therapy for individual patients based on their unique genetic profile. The study will focus on the following key aspects: genetic factors influencing the prognosis of implant treatment; diagnostic tools for screening high-risk populations; omics profiling in osseointegration; and personalized dental implant therapy. The review will also emphasize the importance of integrating omics sciences with advanced bioengineering technologies to enhance bone formation and regulate osteogenesis by elucidating the genetic and epigenetic signaling cascades involved in dental implantology.
Material and methods
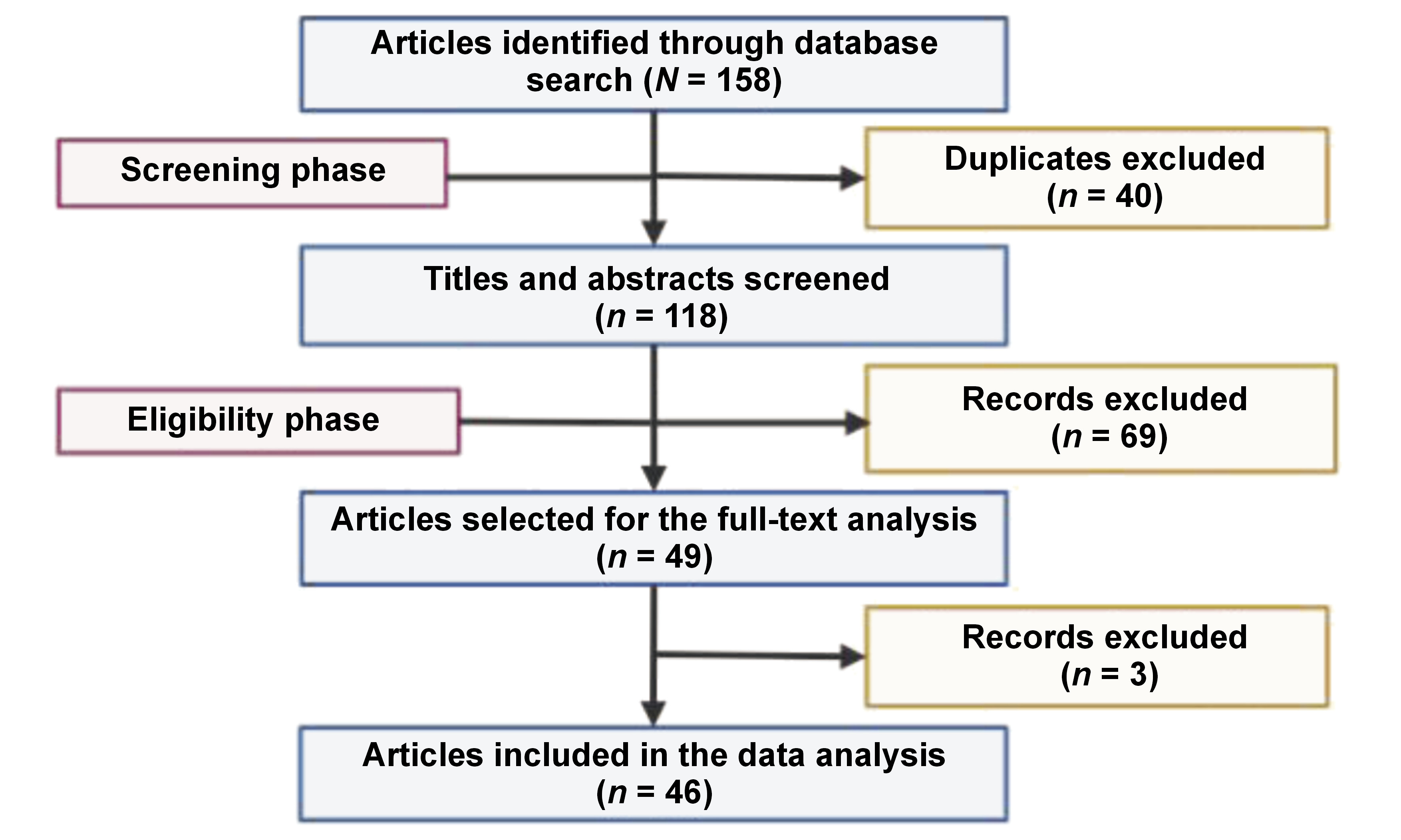
The included studies predominantly focused on genetic and epigenetic contributors to implant failure, with an emphasis on osseointegration. These comprised original research, clinical studies and relevant reviews that examined molecular mechanisms, gene polymorphisms, epigenetic modifications, and the application of omics technologies within the domain of dental implantology. Prominent electronic databases like PubMed®, Scopus, EMBASE, and Web of Science were used to retrieve articles published in the English language during the 16-year period from 2008 to 2024. The time restriction was implemented to preclude the introduction of inaccurate, questionable or outdated concepts while concurrently facilitating the comprehension of the contemporary perception of genetics and its relevance in implant failure. Studies were excluded if they focused solely on mechanical or prosthetic factors, without addressing genetic aspects, or if they lacked peer-reviewed content, including conference abstracts, editorials and opinion articles. A combination of Medical Subject Headings (MeSH) terms such as “genetic markers”, “implantogenomics”, “genetic polymorphism”, “genetic factors”, “epigenetics”, “peri-implantitis”, “implant failure”, “dental implant”, “precision medicine”, “epigenetic mechanisms”, “DNA methylation”, “histone modification”, and “omics” were used with the Boolean operators to curate the data. Duplicates and methodologically weak studies were excluded during the screening process.
The total number of articles retrieved from 4 online databases was 158. During the screening phase, 40 articles were identified as duplicate entries and were hence removed from the study. In the eligibility phase, 118 records were reviewed, of which 72 were excluded due to deviation from the intended study objective. Finally, 46 articles were included in the review (Figure 1).
Results and discussion
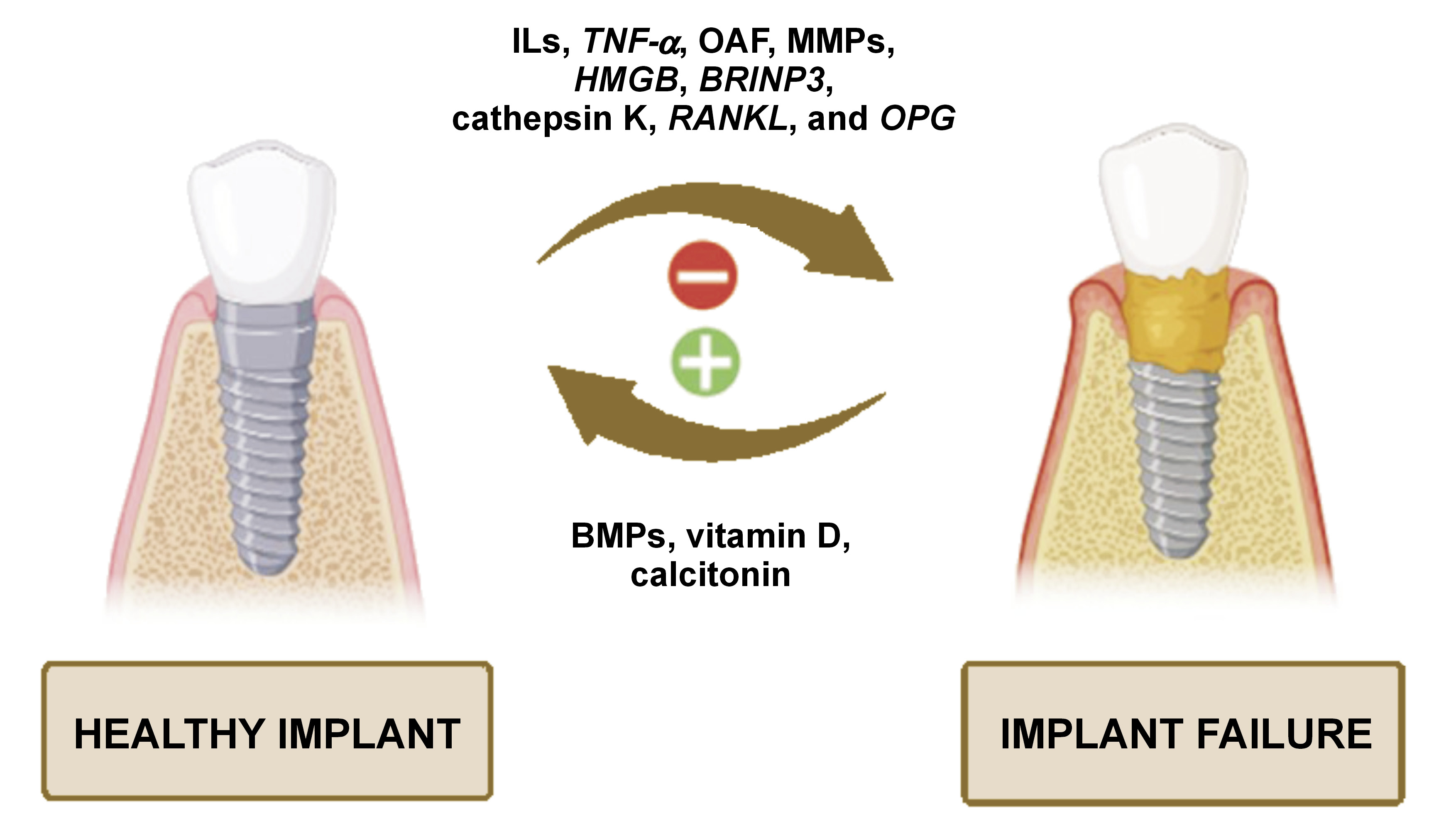
Peri-implantitis and periodontitis have shown similar clinicopathological features, involving soft tissue damage, infection and bone loss.9 An inflammatory process is a notable problem in dental implant patients, as it spreads rapidly and more profoundly around an implant as compared to natural teeth. Therefore, more emphasis should be placed on genes associated with immune/inflammatory responses to foreign bodies.6
Wear of the implant surface results in debris-mediated implant loosening, which is one of the main causes of implant failure. This process is referred to as osteolysis. The particles shed from titanium implants trigger a more robust immune response from macrophages when compared to particles derived from supplementary substances used in implant restoration. The inflammatory and osteolytic process of peri-implantitis is driven by pro-inflammatory cytokines, involving interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α), released by macrophages. The evidence indicates that titanium particles cause inflammation and osseo-disintegration only in certain individuals receiving implants, which underscores a critical role of the host factor in implant failure.10
Genetic factors influencing the prognosis of implant treatment
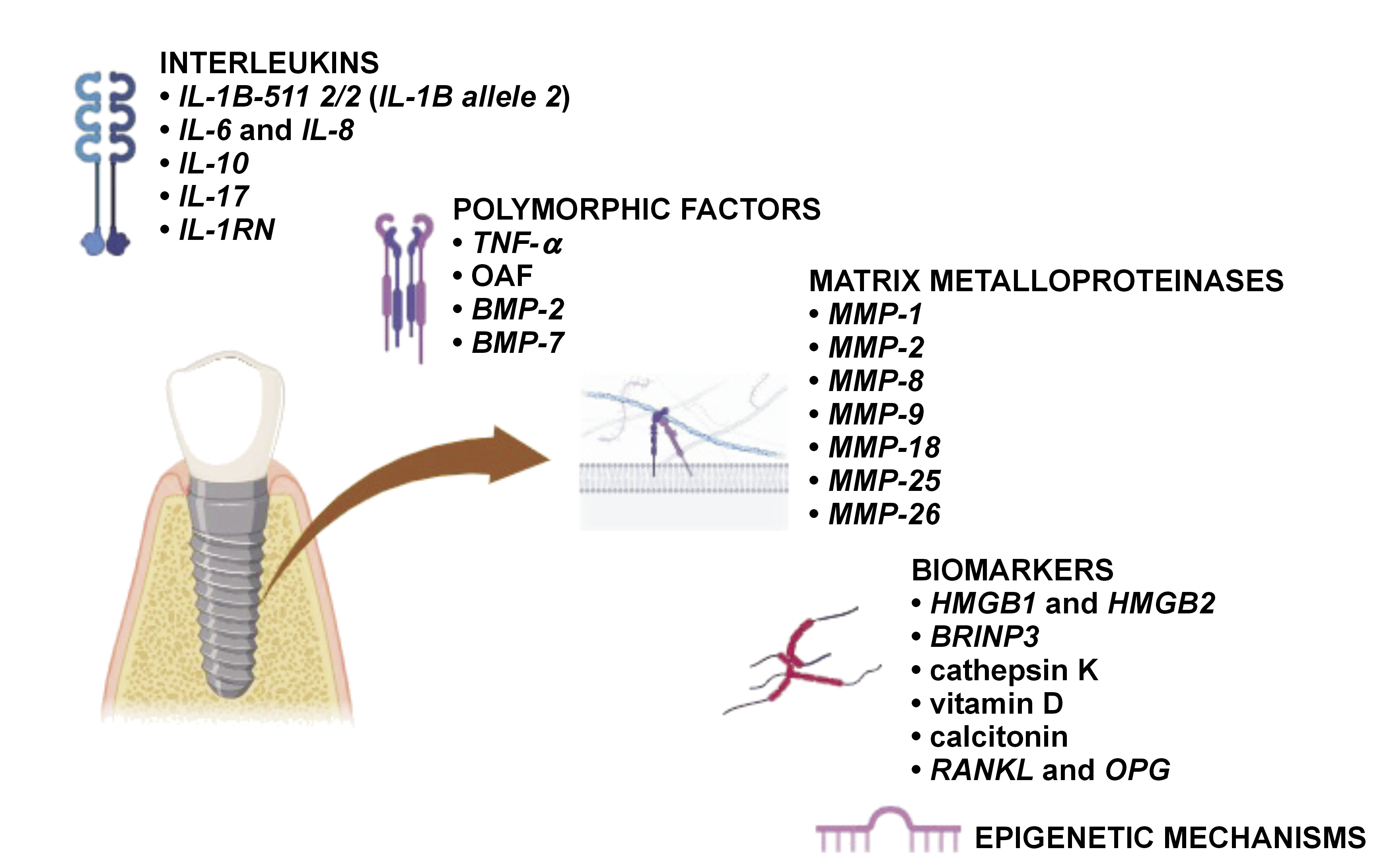
Genetic mediators that play a vital role in the immune/inflammatory reaction of the body can be categorized as follows (Figure 2,Figure 3):
– ILs;
– bone morphogenetic proteins (BMPs), TNF, transforming growth factor (TGF) (TNF-α and TGF-β);
– matrix metalloproteinases (MMPs);
– bone metabolism biomarkers.9, 11
Interleukins
The diagnostic markers of implant failure include IL-1A −889 C/T (rs1800587), IL-1B +3954 C/T (rs1143634), IL-1RN +2018 T/C (rs419598), and TNF-α −308 G/A (rs1800629) genotyping, in vitro IL-1β/TNF-α release assays, and lymphocyte transformation tests.9 Other prognostic markers of peri-implantitis are cathepsin K, receptor activator of nuclear factor kappa-Β ligand (RANKL) or osteoprotegerin (OPG), but further investigations and large clinical trials are necessary to confirm these findings.12 The amalgamation of IL-1 allele 2 (IL-1A −889 and IL-1B +3954) in patients with inflamed periodontal or peri-implant tissues acts as a detrimental factor that exacerbates tissue destruction.13 Table 1 summarizes the impact of different ILs on implant prognosis.
Another non-invasive means of inspecting the host’s reaction in periodontal and peri-implant diseases is the analysis of gingival crevicular fluid (GCF) or peri-implant sulcular fluid (PISF).16
TNF and TGF
Single-nucleotide polymorphisms (SNPs) are minor DNA mutations that influence the process of osseointegration in implants. Inflammatory proteins play a crucial role in both the breakdown of the extracellular matrix (ECM) and the resorption of the alveolar bone.5 Tumor necrosis factor alpha is a proinflammatory cytokine. It serves as the primary mediator in the immune response to Gram-negative bacteria, with TNF-α levels indicating the bacterial load and the severity of inflammation.16 Bone morphogenetic proteins belong to the TGF-β superfamily and have been demonstrated to promote bone ingrowth, facilitate gap healing and enhance implant fixation in various animal studies.18 BMP-2 and BMP-7 are termed human osteogenic proteins. Table 2 presents an overview of various polymorphic factors and their influence on implant treatment.
Matrix metalloproteinases and extracellular matrix remodeling mediators
The extracellular reaction may vary depending on the implant surface, with ECM exhibiting different morphological characteristics across various material surfaces.
Matrix metalloproteinases are a family of highly conserved endopeptidases. These ECM macromolecules contribute to cellular development and morphogenesis. They regulate growth factors, activate cell surface receptors and influence adhesion molecules. Matrix metalloproteinases are involved in various physiological processes, including inflammatory cell activity, wound healing, angiogenesis, and bone formation.20, 21
The family of MMPs consists of 5 groups:
– collagenases: MMP-1, MMP-8, MMP-13, MMP-18;
– gelatinases: MMP-2, MMP-9;
– stromelysins: MMP-3, MMP-10, MMP-11;
– matrilysins: MMP-7, MMP-26;
– membrane-type (MT) MMPs:
• 4 transmembrane MMPs: MMP-14; MMP-15; MMP-16; MMP-24,
• 2 glycosylphosphatidylinositol-anchored MMPs: MMP-17; MMP-25.
The effects of MMPs on ECM remodeling are outlined in Table 3.
Bone metabolism biomarkers
Bone is a metabolically active tissue that undergoes continuous remodeling. This process is driven by the dynamic interaction of osteoblasts and osteoclasts, regulated by a complex network of molecular biomarkers. Table 4 summarizes the impact of these biomarkers on dental implant treatment.
Epigenetic mechanisms
Epigenetics refers to the study of heritable changes in the phenotype that occur without alterations to the underlying DNA sequence. These changes involve modifications to the chromatin structure, which in turn regulate gene expression independently of base sequence variations.29 Environmental factors such as toxins, microbes, stress, diet, and hormones can alter epigenetic patterns, thereby influencing gene activity and cell behavior. These modifications regulate gene expression by either promoting or silencing transcription, blocking mRNA formation or causing protein post-translational modification.30 Key epigenetic mechanisms include DNA methylation, histone modifications and regulation by non-coding RNAs like microRNAs (miRNAs).31 DNA methylation silences gene expression and regulates key bone-related genes. Histone modifications, like acetylation and methylation, also control gene activity. Histone deacetylases (HDACs) have been shown to influence bone health by regulating osteoblasts, osteoclasts and bone mass. MicroRNAs are short non-coding RNAs (18–22 nucleotides) that regulate gene expression by degrading or repressing target mRNAs. Increased levels of miRNAs suppress gene expression, while decreased levels enhance it. Several miRNAs, such as miR-23a, miR-34c and miR-133a, directly influence bone formation by targeting runt-related transcription factor 2 (RUNX2), a key transcription factor in the differentiation of osteoblasts.32 Table 5 provides an overview of key genetic factors and their respective roles at the molecular and biochemical levels in implant integration and failure.
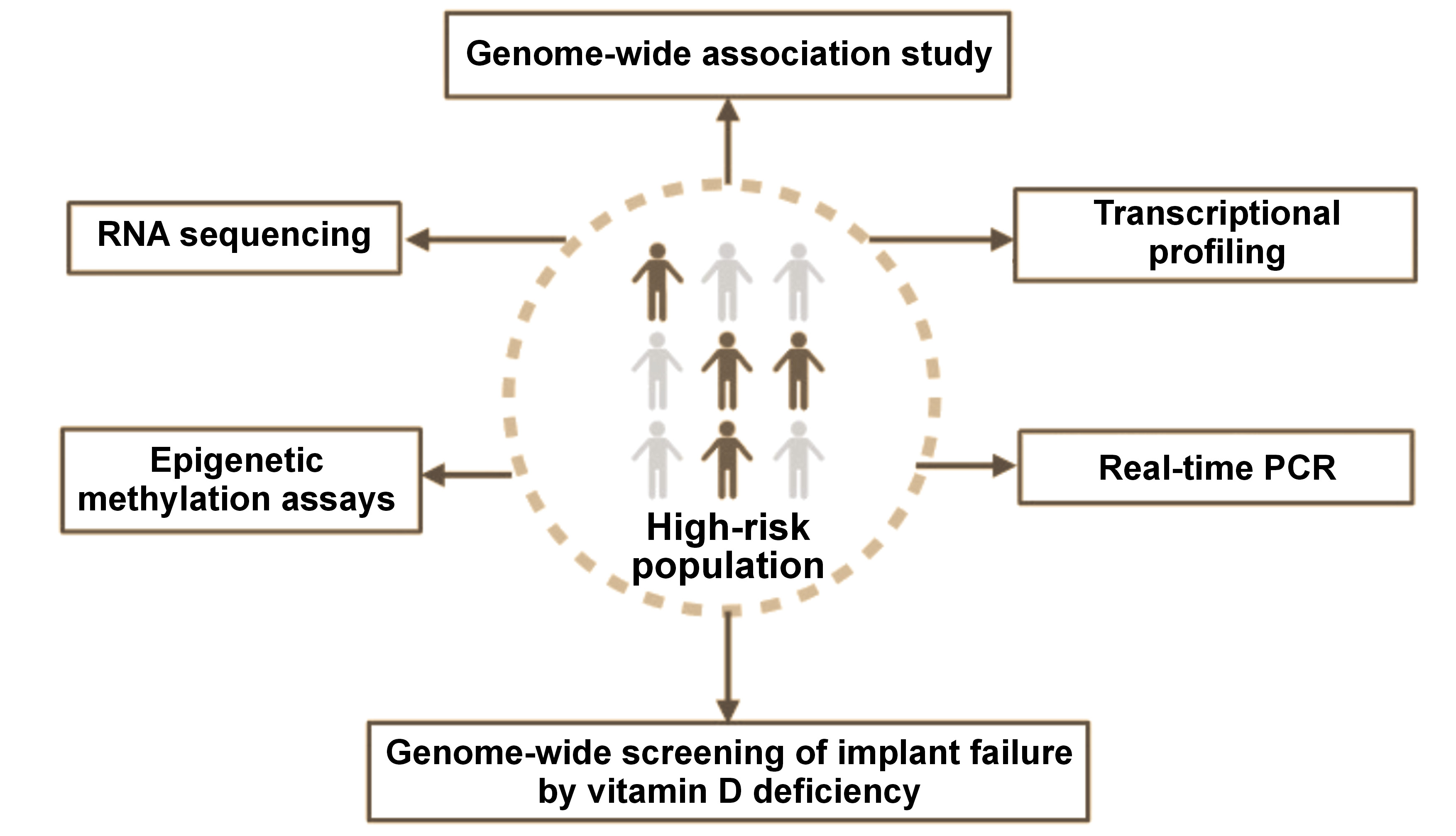
Screening of patients at high risk of implant failure
Some of the diagnostic interventions for the identification of individuals at high risk of implant failure include (Figure 4):
1. Genome-wide association study: It examines the link between SNPs and traits, with a particular focus on major diseases, by comparing the DNA of individuals with different phenotypes related to a specific trait or disease. Participants are divided into 2 groups: those with the disease (cases); and similar sample without the disease (controls). This method, known as the phenotype-first approach, identifies SNPs in which one allele appears more frequently in the disease group. When a SNP demonstrates a significant association with a specific condition, it is considered to indicate a genomic region that could impact disease risk.36
2. Transcriptional profiling of osseointegration: It highlights the complexity of bone healing, a process that involves interwoven biological stages such as inflammation, osteogenesis and angiogenesis. A comparison of gene expression profiles associated with wound healing and those observed at the post-implant site elucidates the natural delays between gene expression, protein translation and tissue maturation. The histological data indicates that the selected time points for transcriptional analysis effectively capture key early transcriptional events that are crucial in the process of osseointegration.37
3. Real-time polymerase chain reaction (PCR): Diagnostic qualitative PCR is used for a rapid detection of disease-specific nucleic acids, while quantitative PCR measures both the presence and the quantity of a particular DNA sequence in each sample. Both quantitative PCR and DNA microarrays are cutting-edge techniques for the analysis of gene expression, offering researchers a more profound understanding of molecular processes. This understanding supports the development of advanced therapeutic prosthetics for dental implant treatments and applications in tissue engineering biology.38
4. RNA sequencing: Next-generation sequencing (NGS) is used to identify and quantify RNA in a biological sample at a specific point in time, enabling the analysis of the dynamic cellular transcriptome.39
5. Genome-wide screening of implant failure by vitamin D deficiency: Genome-wide microarray analyses of implant osseointegration suggest that the unique microenvironment created by implant placement significantly influences multiple gene expression networks, potentially involving peripheral circadian rhythm pathways. Notable interactions between the NPAS2 gene and cartilage matrix genes have led to a proposed model in which bone marrow mesenchymal cells, through a circadian rhythm-related mechanism, initiate ectopic synthesis of cartilage matrix molecules such as type X collagen without forming actual cartilage tissue at the implant site. Additionally, vitamin D deficiency disrupts these processes, impairing effective bone formation and implant integration.40
6. Epigenetic methylation assays: These techniques are used to examine changes in DNA methylation, an important regulatory mechanism that alters gene expression without modifying the underlying DNA sequence. Epigenetic methylation assays facilitate the assessment of the impact of environmental and external factors on gene activity, which contributes to diverse biological processes and disease development.41
The presented molecular techniques are not currently part of routine clinical practice in implant dentistry. However, they are emerging tools with the potential for future applications as the field of precision diagnostics continues to evolve.
Clinically available tests for genetic screening related to dental implants or periodontal diseases include:
1. MyPerioID® IL-6: saliva-based genetic screening tool that identifies variations of the IL-6 gene, a critical marker of inflammation. By detecting these genetic predispositions, the test enables the assessment of an individual’s risk for developing severe periodontal disease42;
2. TNF-α test: TNF-α (G-308A) gene polymorphism has been investigated for its potential association with implant failure and peri-implantitis, as it may influence inflammatory responses. However, research findings have been inconsistent, with some studies showing a possible link and others finding no significant association. Genetic testing for TNF-α variations has yet to be incorporated into standard dental implant planning, as clinical factors like patient health, oral hygiene, bone quality, and surgical technique remain the primary predictors of implant success43;
3. IL-6 test: measures salivary IL-6 levels, serving as a biomarker for periodontitis, a major risk factor for dental implant failure. Elevated IL-6 levels indicate increased inflammation and help identify individuals with a higher risk for periodontitis or implant complications. The protocol entails the collection of a saliva sample, the analysis of IL-6 concentrations, and the assessment of the inflammatory status based on the obtained results. Although it is not a genetic test, the IL-6 test provides valuable insight into a patient’s risk for implant failure due to inflammation and bone loss44;
4. GenoType Periodontal Susceptibility Test (PST): a screening tool for IL-1A and IL-1B gene variations that have been linked to an increased risk of severe periodontitis. A positive result (PST+) indicates higher susceptibility to periodontal disease. While useful for assessing periodontal risk, it is not routinely used in dental implant planning, and evidence supporting its role in predicting peri-implantitis remains limited.45
5. PerioPredict: genetic risk assessment tool that is used to evaluate moderate to severe periodontal disease by analyzing IL-1 gene variations, a key factor in inflammation. While it may offer insights for implant planning, it is not specific to dental implants and has shown mixed results in clinical studies. The clinical examination remains the primary method for assessing the periodontal risk. PerioPredict should be used as a complementary tool in conjunction with other clinical evaluations during treatment planning.46
Building upon the use of clinically available genetic screening tests, omics profiling is now being explored to gain deepe insights into the mechanisms underlying osseointegration.
Omics profiling in osseointegration
Omics is a branch of biology that encompasses fields like genomics, proteomics, transcriptomics, and metabolomics. The primary goal of omics sciences is to identify, characterize and quantify the diverse biological molecules that contribute to the structure, function and dynamics of cells, tissues or organisms.47
Omics technologies have been utilized in distinct pre-clinical studies to understand the early and late molecular events occurring during osseous formation.48 Identifying the genes and proteins that affect osseointegration is essential in order to reduce the healing time associated with implant surgery and improve clinical outcomes in patients with local or systemic conditions that impair bone metabolism.49
Studies have shown that an early stage of osseous wound healing was associated with enhanced chemokine, NF-κB, TNF-α signaling pathway, and angiogenesis-related pathways. In the latter stages, an increased expression of mitogen-activated protein kinase (MAPK), Wnt pathways and proteins associated with ECM remodeling and bone mineralization was observed.50, 51, 52, 53
A few in vitro studies were conducted to evaluate the impact of different implant surfaces on osteogenic markers, thereby influencing the rate of osseointegration. Moderately rough surfaces exhibited elevated levels of osteogenic markers when compared to polished surfaces.54 The hydrophilicity of the implant surface further amplified osteogenesis by positively modulating osteogenesis- and angiogenesis-related pathways (e.g., vascular endothelial growth factor (VEGF), MAPK and BMP pathways).55
Personalized dental implant therapy
The integration of regenerative approaches to customize the implant therapy as per the patient’s individual needs has given rise to a new concept termed implantogenomics or implantomics.47
Considerable efforts have been made to create bioactive surface coatings that emulate the biochemical composition and structural characteristics of human bone at the nanoscale. Taking insights from recent omics research, new experimental coatings are currently under development. These coatings are engineered to incorporate targeted drugs, agents, proteins, and growth factors that enhance implant stability by supporting the natural process of osseointegration.56 Preclinical studies have shown that coating dental implants with ECM proteins can enhance peri-implant bone formation. De Barros et al. reported increased bone volume and mineralization with collagen type II/chondroitin sulfate coatings in a canine model.57 Meng et al. reviewed 34 studies on biomolecular coatings for titanium dental implants, mostly in animal models, and found that growth factors, peptides and ECM proteins may support early stages of bone integration.58 However, the authors noted inconsistent results and highlighted the need for clinical studies in humans.58 Hasani-Sadrabadi et al. developed a layer-by-layer surface treatment for titanium implants incorporating BMP-2-mimicking peptides and gentamicin to enhance osseointegration and antibacterial activity.59 Using a polydopamine coating to support nanolayer formation, the modified surfaces enabled sustained release of bioactive agents. In vitro and in vivo studies have demonstrated improved cytocompatibility, osteogenic differentiation and peri-implant bone integration, suggesting promising applications in the fields of dental and orthopedic implantology.59 Zhou et al. developed a 3,4-dihydroxyphenylalanine (DOPA)-based peptide coating (DOPA-P1@P2) for titanium implants to address aseptic loosening by promoting staged bone regeneration.60 The coating sequentially modulated inflammation, angiogenesis and osteogenesis through specific bioactive peptides. In vivo, it significantly improved push-out strength, bone volume and bone-to-implant contact compared to TiO2 controls, suggesting its strong potential for enhancing implant osseointegration.60
Due to ethical restrictions and an increased prevalence of implant failure, there has been a paucity of in vivo studies investigating the constituents of the host genetic susceptibility that influences biological complications in implant placement.49 However, omics technology, which offers a comprehensive understanding of biomaterials, marks a major advance in biomedical science, which will significantly advance the growth of tailored and personalized medicine in implant dentistry.61 Genetic screening offers potential for personalized dental implant therapy, as it enables the customization of implant design and treatment plans based on a patient’s genetic profile. However, challenges such as the complexity of genetic data interpretation, the high cost of testing, and the need for further research to confirm the clinical relevance of genetic markers limit its routine use.
Conclusions
Implant failure is a serious concern in the prognosis of dental implants. Even though the mechanisms underlying implant loss are well-defined, they vary depending on a case. Determining the underlying direct or indirect cause of implant failure is of the utmost importance.
A single-nucleotide polymorphism of pro-inflammatory mediator genes might influence their expression intensity or amino acid sequence, thereby affecting the host inflammatory response. Some SNPs have been correlated with implant loss and determined as probable genetic risk factors for implant failure. Studies on the subject have contributed to the redefinition of prospective targets for successful screening, prevention and maintenance of dental implants. The application of insights from omics sciences has the potential to drive further advancements in personalized dental implant therapy, promoting long-term clinical success.
Ethics approval and consent to participate
Not applicable.
Data availability
Not applicable.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.