Abstract
The hyoid bone exhibits potential sex-based variations and is implicated in the severity of obstructive sleep apnea (OSA). Sex-specific comparisons are lacking. The present meta-analysis aimed to address this gap.
The Embase, MEDLINE and Web of Science databases were searched. The inclusion criteria were as follows: studies that reported the measurements of the hyoid bone–mandibular plane distance (HMP), demonstrated in cephalometric imaging (CEPH) in patients with OSA of both sexes, involving a polysomnography (PSG) examination with the apnea–hypopnea index (AHI), as well as information on the body mass index (BMI) and age. The exclusion criteria comprised reviews, meta-analyses and case reports. The risk of bias was assessed with the use of the Scottish Intercollegiate Guidelines Network (SIGN) checklist. Statistical analysis was conducted using Comprehensive Meta-Analysis software (CMA) and IBM SPSS Statistics for Windows.
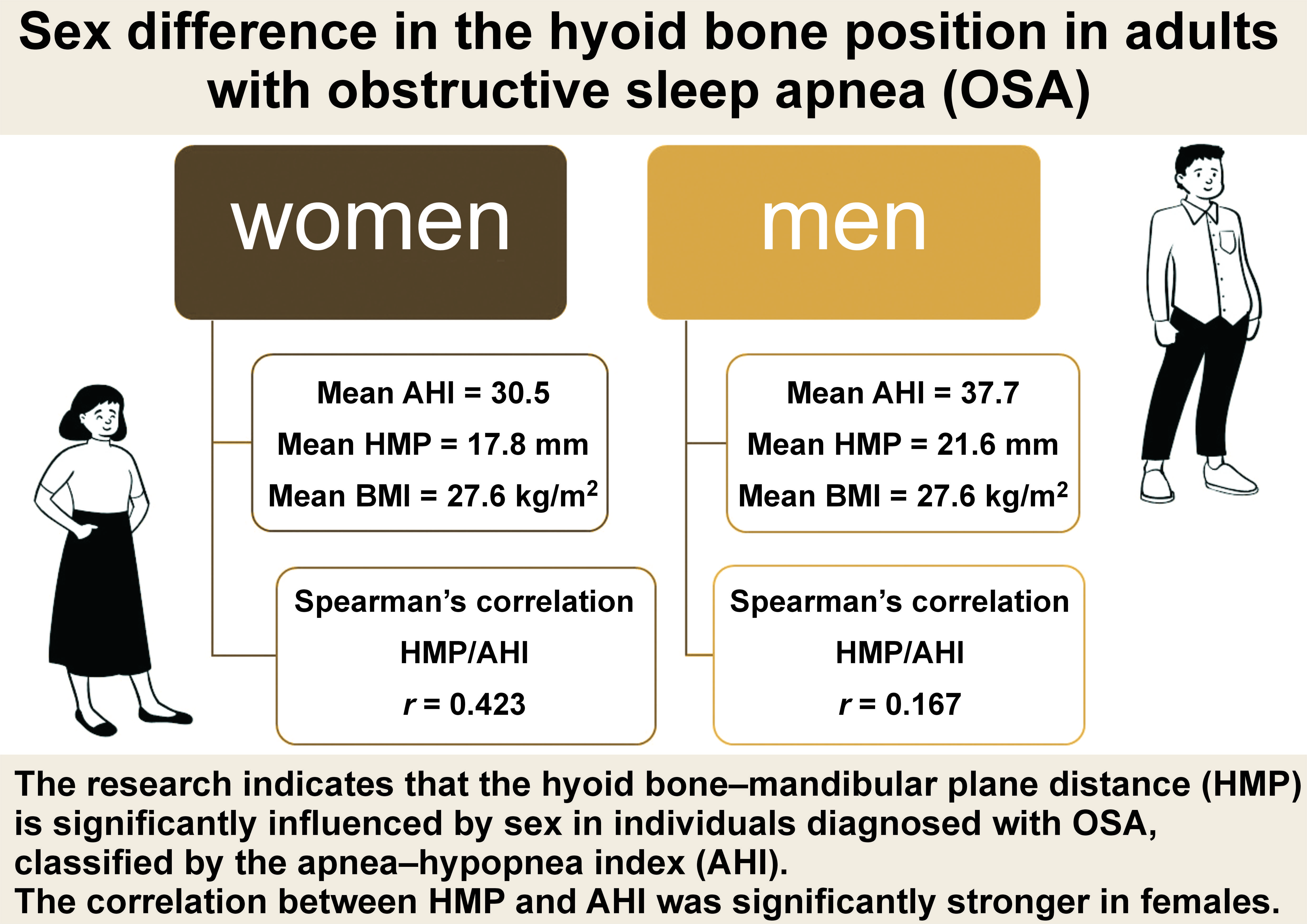
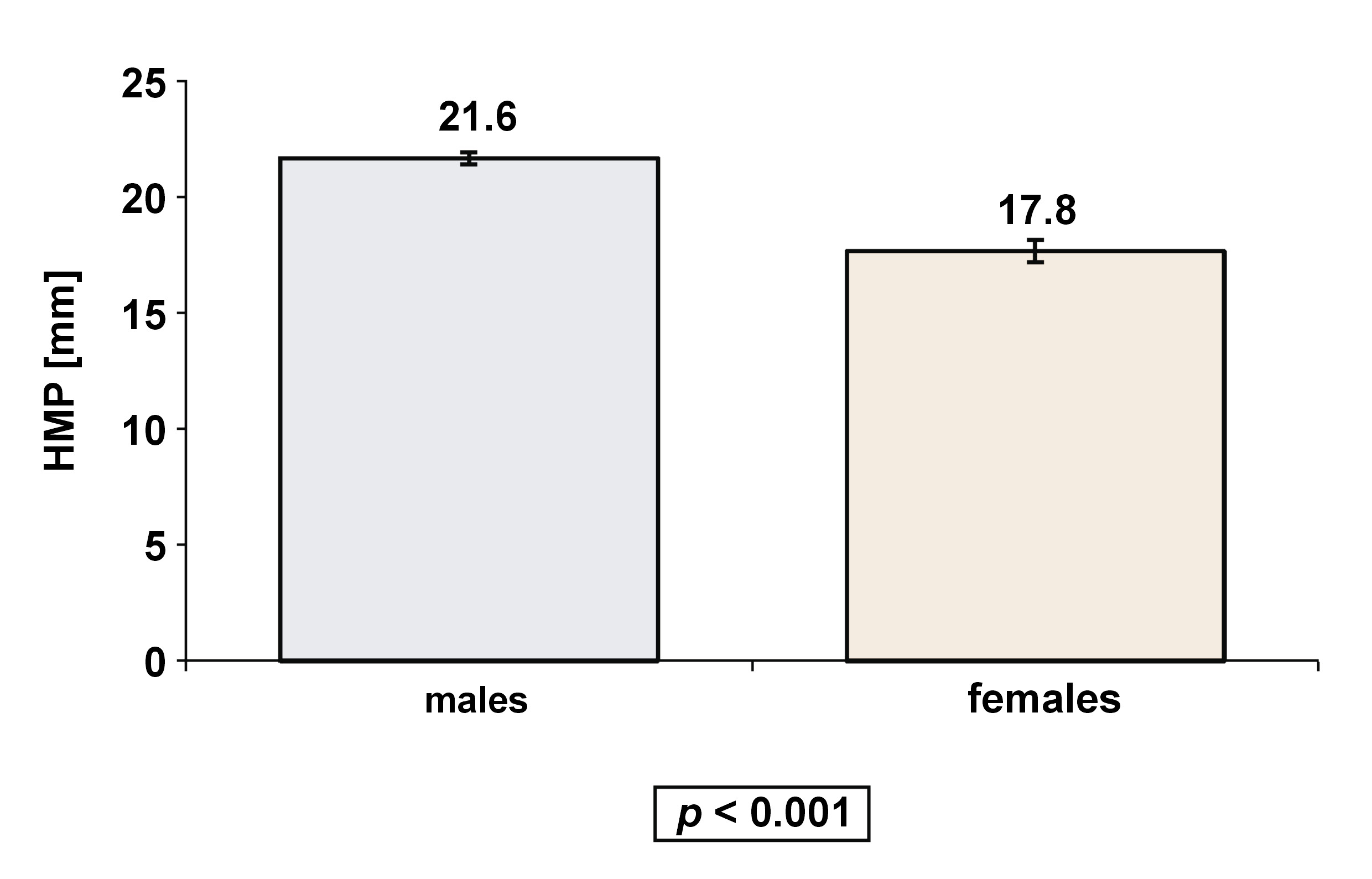
Seven observational studies with 718 adult patients (515 males and 203 females) met the inclusion criteria. The mean HMP value was 20.5 ±3.8 mm, with a significant difference observed between males (21.6 ±3.3 mm) and females (17.8 ±3.7 mm) (p < 0.00001). The correlation between HMP and AHI was significantly stronger in females – 2.5 times higher than in males (r = 0.423 vs. r = 0.167, respectively).
Although a standard range of the hyoid bone position for healthy adults and elderly individuals is currently lacking, sex significantly affects the anatomical variation of the hyoid mandibular position in patients with OSA. It is crucial to identify distinct OSA endotypes by sex to ensure accurate diagnosis and treatment planning, which could lead to sex-specific therapeutic strategies.
Keywords: obstructive sleep apnea, sex differences, hyoid bone position, cephalometric imaging
Introduction
Sex-based medicine is a branch of medical science that focuses on understanding and addressing the physiological and biological distinctions between males and females.1, 2 This field recognizes that the biological differences associated with sex, including anatomical variations and genetic factors, can significantly impact health outcomes and responses to medical treatment.2, 3 Sex-based medicine advances our understanding of how these differences influence disease manifestation and progression, and treatment efficacy.1 By considering sex-specific factors in research, diagnosis and treatment protocols, healthcare professionals aim to provide more tailored and effective medical care for both men and women.2, 4
Some evidence suggests significant sex-based differences in the hyoid bone position,5 morphology6 and volume.7 The hyoid bone is a unique, horseshoe-shaped structure situated in the anterior midline of the neck, constituting a distinctive feature in the human skeletal system.8 Unlike other bones, the hyoid bone does not articulate with any other bone. Instead, it is suspended by ligaments and muscles.8, 9 It is comprised of a central body and 2 pairs of processes extending from its ends – the greater and lesser horns. The hyoid bone is a crucial anchor for various muscles and ligaments involved in the intricate biomechanics of the head and neck.8, 9 It also plays a vital role in supporting the upper airway, as it is positioned at the 3rd cervical vertebra (C3) level. It contributes significantly to essential functions, such as speech, mastication and swallowing.10
Obstructive sleep apnea (OSA) is the most common sleep disorder in the adult population (prevalence of 6–17%).11 Men have a higher rate of OSA than women. This sex difference persists even when accounting for the age and body mass index (BMI) differences between men and women. The risk of OSA increases with age for both sexes.12
Obstructive sleep apnea involves a decrease or complete halt in airflow despite an ongoing effort to breathe.12 It occurs when the muscles at the floor of the mouth, the suprahyoid muscles (including mylohyoid, geniohyoid, digastric, and stylohyoid muscles), relax during sleep, causing the soft tissue in the throat to collapse and block the upper airway.12 The etiological factors of OSA include the craniofacial anatomical features, such as mandibular size, mandibular body length and the tongue volume,13 the accumulation of fatty tissue in the parapharyngeal area and increased body weight, which may decrease the upper airway diameter, thus favoring its collapse.13 Impaired neural control and upper airway myopathy can also increase the risk of OSA.14 Untreated OSA is related to 5 major cardiovascular diseases: hypertension; heart failure; atrial fibrillation; coronary artery disease; and stroke.15, 16, 17 Recently, it has been found that different biomolecules (calcium (Ca), magnesium (Mg), vitamin D, and uric acid), as well as serum neuronal PAS domain protein 2 (NPAS2) metabolic dysregulation may be implicated in the OSA etiology.18, 19
The gold standard for diagnosing OSA is a sleep study.12 The apnea–hypopnea index (AHI) classifies the severity of OSA into 3 categories: mild; moderate; or severe.20, 21 Several imaging methods, such as video fluoroscopy, cephalometric imaging (CEPH), computed tomography (CT), and magnetic resonance imaging (MRI), static or dynamic, may aid clinicians in better recognizing the individual craniofacial anatomical features that may be the cause of OSA, thus helping in diagnosis and personalized treatment planning.22 Each kind of imaging presents advantages and disadvantages. Cephalometric imaging remains a valuable tool for assessing skeletal structures and providing an initial evaluation of the upper airway.
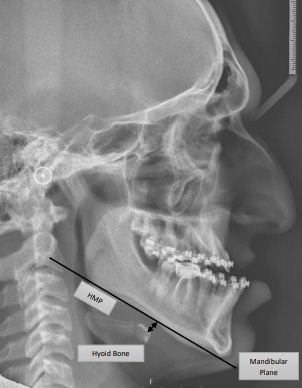
One of the anatomical features, the inferior-dorsal position of the hyoid bone, which is measured via CEPH,23, 24, 25 and expressed as the hyoid bone–mandibular plane distance (HMP) in millimeters, is suggested to be of importance in some patients with OSA.26, 27, 28 One of the parameters for measuring the hyoid bone position in CEPH is the perpendicular distance from the most superior-anterior point on the body of the hyoid bone (H) to the mandibular plane (MP), which is constructed by connecting the lowest point on the lower border of the mandibular body (gnathion) and the lowest point on the lower border of the mandibular ramus (gonion), the reference point on the C3 being the most inferior-anterior point on the body of the C3 (Figure 1).29 The average HMP value in the healthy population is 9.03 ±3.92 mm, whereas it amounts to 22.81 ±6.76 mm in OSA patients.30 It has been found that HMP is longer in patients who suffer from severe OSA as compared to those who do not.31According to a meta-analysis conducted by Neelapu et al., there is an average difference of 4.0–6.6 mm in HMP between patients with OSA and those without it (controls), with OSA patients having a longer HMP.28
Surprisingly, the abovementioned meta-analysis and other cited studies did not perform any comparison between males and females,28, 30, 31 even though several significant sex-based differences in the OSA prevalence, clinical presentation and management are supported by research.32
Considering the fact that sex plays a role in OSA and affects the hyoid bone position, as well as the reported greater HMP values among patients with OSA, we investigated in the present systematic review and meta-analysis whether there were significant sex-related differences in the hyoid bone position in patients with OSA.
Methods
We developed a review protocol according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement.33 The study protocol was registered with PROSPERO before initiating this systematic review and meta-analysis (ID: CRD42023446388).
Identification and selection of studies
The electronic databases Embase, MEDLINE and Web of Science were searched with regard to the period from 1946 to February 2023.
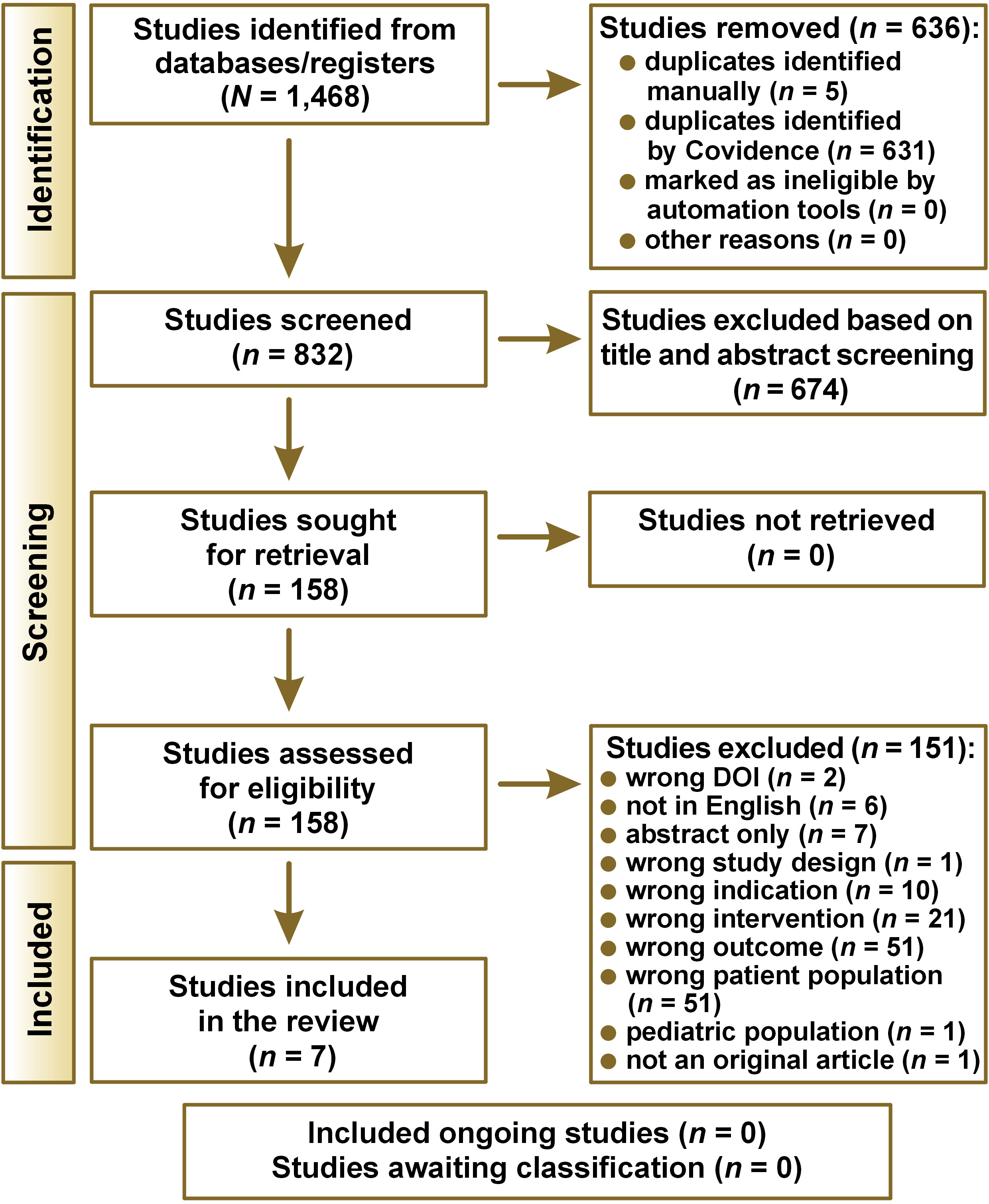
A comprehensive search was conducted across the databases on February 15, 2023, focusing on the ‘hyoid bone’ and its association with sleep-disordered breathing, including ‘sleep apnea’. The queries yielded 637 results from Embase, 473 results from MEDLINE and 358 results from the Web of Science. Thus, there was a total of 1,468 initial records. After removing duplicates, 832 unique records were identified and uploaded into the Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia; https://www.covidence.org). Two independent reviewers (D.G.-A. and A.E.-P.) screened the titles and abstracts of all the articles to assess the eligibility of each study.
Eligibility criteria
To be eligible, a study had to be in English; it had to be an observational, cross-sectional or clinical study reporting HMP in OSA patients of both sexes, using CEPH; it had to involve a polysomnography (PSG) examination (at a cut-off value of the apnea–hypopnea index (AHI) ≥5), and provide information on the patients’ BMI, age and sex. Reviews, meta-analyses and case reports were excluded. Both reviewers performed the screening and assessments, discussing their progress and decisions.
Outcome measures
The outcome measures in this study were AHI as a means to define the presence of OSA and CEPH as a means to determine HMP in millimeters.
Data extraction
After extracting the data, we further disqualified articles by reading the titles and abstracts. In the next step, the reviewers read the texts in full. In case of disagreement, they discussed the issues with a third reviewer (T.G.). Authors’ names, the journal name, the year of publication, the country, and the method used to diagnose OSA were registered. The reviewers analyzed the following parameters: the sample size (the total number of cases with OSA); the patients’ mean age and their sex (the number of male and female patients suffering from OSA); the patients’ mean BMI; and the mean HMP for each sex. To evaluate the correlation between HMP and OSA for both sexes, statistical analysis was performed, as detailed below.
Assessing the risk of bias
To assess the risk of bias, we used the NIH quality assessment tool – the Scottish Intercollegiate Guidelines Network (SIGN) checklist (National Institutes of Health (NIH), Bethesda, USA) to estimate the quality of observational cohort and cross-sectional studies (supplementary material available from the corresponding author on request). Two independent reviewers (D.G.-A. and A.E.-P.) employed the following methodological criteria to assess the risk of bias in each of the eligible studies: the research question or objective clearly stated; the population specified and defined; a participation rate of eligibility (>50%); clear inclusion/exclusion criteria; sample size justification; the exposure(s) of interest measured prior to the outcome(s) being measured; the timeframe sufficient for an association between the exposure and the outcome; the examination of different levels of the exposure as related to the outcome; the definition of the exposure measures, and their validity and reliability; the binding of the exposure to the definition of the outcome measures, and their validity and reliability; and the measurement and statistical adjustment of the critical potential confounding variables.
Each reviewer completed the SIGN/NIH checklist for the included studies, and determined the overall risk of bias, rating it as low (a score of 9–12 methodological points), moderate (a score of 5–8 methodological points) or high (a score of 0–4 methodological points). Any disagreement was resolved through discussion with a third reviewer (T.G.). The reviewers contacted the authors of the publications for clarification in case of unclear or missing information.
Statistical analysis
Meta-analysis was performed using primary outcome measures when there were 5 or more studies with a low to moderate risk of bias, and similar assessment and measurement techniques. The results for the eligible studies were pooled using the Cochrane ReviewManager (RevMan; https://training.cochrane.org/online-learning/core-software) via a random effect. The one-way analysis of variance (ANOVA) was conducted using the Comprehensive Meta-Analysis software (CMA), v. 4.0 (Biostat, Inc., Englewood, USA; https://meta-analysis.com). This analysis examined differences in the position of the patients’ hyoid bone by sex, considering additional parameters, such as AHI, BMI and age. In addition, an attempt was made to examine differences in those parameters between the patients with OSA and those without OSA; however, due to the paucity of studies regarding the population without OSA, it was impossible to conduct a statistical analysis with sufficient validity and power.
We built a data model for multivariate analysis using IBM SPSS Statistics for Windows, v. 23.0. (IBM Corp., Armonk, USA), applying the weight function according to the number of observations in each study and each sex group. Some of the one-way tests were repeated with a tool more familiar to the researcher (IBM SPSS Statistics for Windows) to analyze the findings previously examined in CMA. Spearman’s correlations were determined with regard to HMP, AHI and BMI for each sex separately. Then, a generalized linear model analysis was performed for HMP as a dependent variable based on AHI, BMI, age, and sex.
Confidence in cumulative evidence
For each outcome, we evaluated the level of confidence in the evidence accumulated from all sources by following the guidelines outlined in GRADE.34, 35, 36, 37, 38 We conducted a comprehensive assessment, considering various factors, such as the risk of bias, the consistency of the results, the effect size, and the sample size. Based on this analysis, we assigned each outcome an overall confidence level – high, moderate, low, or very low.
Results
Study selection
The literature search yielded 1,468 studies, of which 636 duplicates were removed, leaving 832 studies that were screened by title and abstract. After that, only 158 studies remained relevant. After reading the texts in full, 7 articles were selected and included in the present study. Out of the 151 studies that were rejected based on full text, the majority were excluded due to wrong patient population, outcome and intervention. The review process flow chart is shown in Figure 2.
Study characteristics
The eligible studies included in the analysis were all observational and cross-sectional, except for one retrospective cohort study.39 They used lateral cephalometric radiographs as an outcome measure for patients with OSA. The characteristics of each study are presented in Table 1.
Assessment of the risk of bias
This systematic review assessed the risk of bias in each study. Among the cross-sectional and cohort studies, 6 studies were rated as having a low risk of bias,40, 41, 42, 43, 44, 45 while one was rated as having a moderate risk of bias (Table 2).39 However, none of the studies provided details regarding the duration of the patients’ exposure to OSA, and only 2 studies had the HMP assessor blinded to the severity of OSA.41, 44 Only one study provided a clear justification for the sample size.40 The main confounders identified in most of the included studies were AHI, BMI and age.
Participants
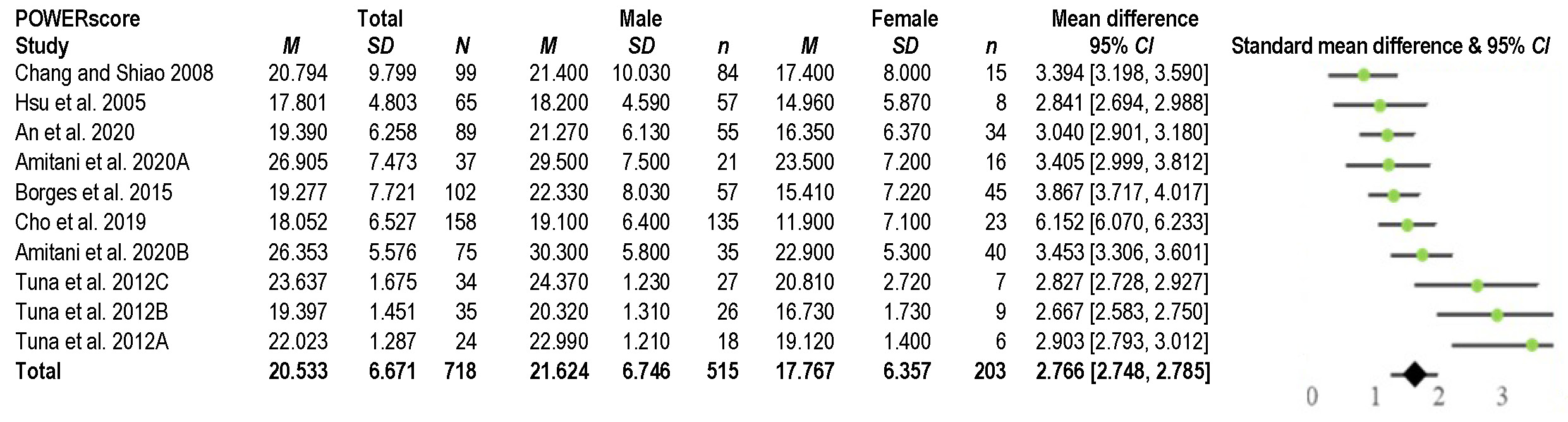
The study examined data from 7 studies on OSA in 718 adult patients. The average age of the patients was 49.0 ±5.9 years. Out of all OSA patients, 515 (72%) were males with an average age of 47.5 ±4.8 years, while 203 (28%) were females with an average age of 53.0 ±6.7 years. The study noted a significant age difference between men and women (p < 0.00005), but there was no significant difference in BMI (p = 0.9771) (Table 3). These factors were considered in the analysis of the main outcome measure.
Main findings
As shown in Table 3, the average AHI value was 37.5 ±10.3, with a significant difference between males (37.7 ±10.3) and females (30.5 ±10.3) (p < 0.00001). For HMP, the mean value was 20.5 ±3.8 mm, with a significant difference between males (21.6 ±3.3 mm) and females (17.8 ±3.7 mm) (p < 0.00001), as shown in Figure 3. These results stayed very similar after neutralizing the effect of age and BMI (Figure 4).
The results of Spearman’s correlation analysis (Table 4) showed a significant moderate positive correlation between HMP and AHI in women (r = 0.423; p < 0.00001). However, the correlation was weak and positive in men (r = 0.167; p < 0.00001). Additionally, a significant weak positive correlation was observed between HMP and BMI in both females (r = 0.219; p < 0.01) and males (r = 0.328; p < 0.001). A significant moderate positive correlation was found between BMI and AHI in females (r = 0.568; p < 0.0001), while a weak positive correlation was observed in males (r = 0.304; p < 0.0001).
Table 5 presents findings from the linear regression analysis investigating the influence of sex on HMP, independent of age, BMI and AHI, and the correlation between sex and AHI. The analysis indicates a significant impact of sex on HMP. No significant relationship was observed beyond the main effect of sex and OSA.
Confidence in cumulative evidence
According to the GRADE guidelines,37 high-quality evidence supports the observation that there are sex differences in the HMP of patients with OSA (95% confidence interval (CI): 2.75, 2.79; p < 0.0001; GRADE tool: high-evidence profile). Additionally, there appears to be a stronger correlation between HMP and AHI in females with OSA than in males with OSA. Heterogeneity was calculated using the I2 statistic, with I2 values of 30–50% indicating moderate heterogeneity, 51–75% suggesting substantial heterogeneity, and >75% indicating considerable heterogeneity.
Discussion
This is the first comprehensive review and meta-analysis to explore sex-related differences in the hyoid bone position among patients with OSA.
After conducting a comprehensive review of the literature on the airways, OSA and the hyoid bone, we chose the variable HMP as the focus of our meta-analysis, as among the various variables assessed via cephalometric analysis, HMP consistently exhibited a strong correlation with OSA across multiple studies.39, 40, 41, 42, 43, 44, 45 Consequently, we decided to base our study on this specific variable.
The presented results highlight several key findings regarding the relationship between HMP, AHI, BMI, and sex. Firstly, the data reveals significant differences between males and females in the AHI and HMP values. Males exhibited a higher average AHI (37.7 ±10.3) as compared to females (30.5 ±10.3), indicating a more severe degree of OSA in men. Similarly, the mean HMP value was higher in males (21.6 ±3.3 mm) than in females (17.8 ±3.7 mm). These differences persisted even after controlling for age and BMI.
The correlation analysis further elucidated the relationships between these variables. In women, a significant moderate positive correlation (r = 0.423) was observed between HMP and AHI, suggesting that increased HMP values are associated with higher AHI scores, indicative of more severe OSA. However, this correlation was weaker in men (r = 0.167). Additionally, both sexes exhibited a significant but weak positive correlation between HMP and BMI. The body mass index demonstrated a moderate positive correlation with AHI in females (r = 0.568), but a weaker correlation in males (r = 0.304). This finding highlights the potential influence of obesity on OSA severity, particularly in women.
Obesity is thought to contribute to OSA through several mechanisms.46 First, excess fat deposits can accumulate in the airways, narrowing the airway passages. Additionally, obesity can lead to diastolic dysfunction and fat accumulation in the diaphragm muscle, obstructing normal breathing. Moreover, in obese individuals, fat tends to build up in the neck region, resulting in a shorter, thicker neck with a smaller, softer upper airway. This makes the upper airway more prone to collapse or close during sleep, increasing the risk of developing OSA.47 Women have relatively thinner necks on average.39 The dorsal positional migration of the hyoid inside the female neck due to the deposited fat could have a greater effect on the airway pathways, potentially impacting the severity of OSA in females (expressed in AHI) as compared to males, indicating that there may be sex-based clinical relevance to consider. This hypothesis is supported by a better response of females with OSA to a mandibular advancement device (MAD) in comparison with males, especially in severe OSA, but also across a range of AHI thresholds.47 The potential mechanisms underlying the differences between sexes may also be related to the different morphology of the hyoid bone in women and men. In men, the hyoid angle is greater; with advancing age, the hyoid bone moves posteriorly, in rotation, and its position lowers.48 The weakening of the muscles with age may also affect OSA morbidity. Additionally, anatomical differences in HMP between sexes is likely influenced by the complex interplay of sex hormones, developmental processes and aging. While men tend to have a longer upper airway, predisposing them to a higher OSA risk, women benefit from protective mechanisms beyond simple anatomical differences. These mechanisms involve hormonal influences, differences in tissue properties and potentially more efficient neuromuscular responses in the upper airway.48, 49, 50, 51
In a recent systematic review and meta-analysis conducted by Camañes-Gonzalvo et al., focusing on identifying the phenotypic characteristics of responders to oral devices (MAD), it was found that responders, as compared to non-responders, are younger patients with a smaller neck circumference, a lower BMI and a shorter distance from the hyoid bone to the C3,52 which points to the need to take into account, among other factors, the hyoid bone position while considering OSA treatment options.
The linear regression analysis revealed a significant impact of sex on HMP, independent of age, BMI and AHI, and the relationship between sex and AHI. This suggests that biological differences between males and females may contribute to variations in HMP, potentially influencing the risk and severity of OSA.
It is worth noting that while lateral CEPH can provide a basic assessment of the hyoid bone position, MRI offers a more comprehensive and detailed evaluation, enabling a better understanding of the role of the hyoid bone position in the pathogenesis of OSA. Dynamic sleep MRI shows the exact sites and pattern of obstruction while asleep, with no radiation as in the case of CEPH.22 This information can guide treatment decisions, such as implementing surgical interventions (e.g., hyoid suspension or repositioning), or the selection of appropriate oral appliances or myofunctional therapy for managing OSA in specific patients.
Future directions and clinical implications
To ensure the accuracy of the findings of this meta-analysis, it is crucial to establish a standard range of the hyoid bone position (measured through HMP) for healthy adults and elderly individuals, considering sex differences. Furthermore, it is essential to investigate the underlying causes of the downward and backward movement of the hyoid bone in patients with OSA, emphasizing sex-based factors. Such research could lead to a gender-specific approach to treating OSA patients in the future.
Taking both sex and HMP into consideration could potentially serve as a predictor of OSA severity or the treatment response. Clinicians could use this information to evaluate patients’ risk, guide treatment decisions or predict the outcomes of interventions, like the application of MAD. Due to anatomical differences between sexes, women typically require less mandibular advancement than men to achieve the same therapeutic outcome. This suggests that female patients tend to respond more effectively to treatment with smaller degrees of jaw protrusion. Additionally, knowing the position of the patient’s hyoid, myofunctional therapists can design personalized exercise programs. For instance, patients with a lower hyoid position might require more focus on exercises that target the suprahyoid muscles. This can help myofunctional therapists establish a baseline and set specific goals for therapy. Regarding surgical planning, HMP might be a predictor of surgical success, particularly for the procedures aimed at increasing the posterior airway space. The measurement of HMP can inform decisions about the type and extent of surgical interventions. For example, it may influence choices between the mandibular advancement, genioglossus advancement or hyoid suspension procedures. Additionally, understanding sex differences in HMP in OSA patients provides a common reference point for discussions between dentists, ear, nose and throat specialists (ENTs), physical therapists, sleep medicine specialists, and other healthcare providers. Finally, a comprehensive grasp of the intricate pathophysiology of OSA paves the way for significant advancement in treatment. First, it enables the creation of therapies tailored to specific pathophysiological endotypes. Second, it propels the field toward precise sex-oriented medicine, potentially offering patients an alternative to the standard continuous positive airway pressure (CPAP) therapy.
Limitations
A standard range of hyoid bone position (measured through HMP) for healthy adults and elderly individuals, considering sex differences, is currently lacking. The reliance of the study on two-dimensional (2D) imaging techniques, such as lateral cephalometry, for measuring HMP is a limitation, as three-dimensional (3D) imaging methods, like MRI, may provide more accurate and comprehensive assessments of the role of the hyoid bone position in the obstruction of the upper airway.
Conclusions
The results of this study underscore the complex interplay between anatomical factors, such as HMP, and physiological variables, including AHI and BMI, in the context of OSA. Furthermore, the observed sex differences highlight the importance of considering gender-specific factors in evaluating and managing OSA. To ensure the accuracy of the findings of this meta-analysis, it is crucial to establish a standard range of hyoid bone position (measured through HMP) for healthy adults and elderly individuals, considering sex differences. In our review, only two studies reported HMP in healthy individuals in women (12.43 ±8.79 mm) and men (16.69 ±4.54 mm).44, 45 The lack of the healthy individual HMP standard reference emphasizes the need for additional studies that would investigate the HMP value among the healthy population. Based on the GRADE guidelines, high-quality evidence supports the significant role of sex with regard to HMP in OSA patients, with a higher correlation in females.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.