Abstract
Background. Following selective caries removal, the efficacy of bonding to the dentin substrate is questionable due to alterations in mineral content and the quality of remaining dentin, especially in deep proximal cavities.
Objectives. The aim of the study was to evaluate the microtensile bond strength of resin composite to artificially demineralized dentin and sound dentin compared to demineralized dentin that underwent different remineralization protocols. Additionally, the study sought to evaluate the effect of the 2 bonding modes (total-etch/self-etch) on the applied remineralizing agent, and to analyze the failure mode.
Material and methods. The study utilized 30 sound proximal molar surfaces. They were randomly divided into 5 groups based on the remineralization protocols, as follows: sound dentin as positive control (G1); artificially demineralized dentin but without surface treatment as negative control (G2); and artificially demineralized dentin surface groups with surface treatments (sodium fluoride (NaF) solution (G3), nano-hydroxyapatite (nHAp) (G4), and a combination of remineralizing agents (G5)). Different modes of application of the bonding agent (total-etch (B1) and self-etch (B2)) were implemented. Then, a nanohybrid composite was applied and light cured. The microtensile bond strength test was performed, and the failure mode was evaluated.
Results. The application of the total-etch mode resulted in a statistically significant difference between microtensile bond strength values of remineralization protocols. The NaF and nHAp groups (G3 and G4) exhibited the highest values. A statistically significant difference was observed between the microtensile bond strength values of remineralization protocols in the self-etch mode. The positive control, NaF, nHAp, and combined groups (G1, G3, G4, and G5) showed the highest values. The negative control group (G2) in both bonding modes demonstrated the lowest microtensile bond strength.
Conclusions. All remineralization protocols applied to demineralized dentin demonstrated an improvement in bond strength that was equivalent to sound dentin. All remineralized dentin surfaces exhibited a favorable failure mode in comparison to the negative control group.
Keywords: deep demineralized dentin, microtensile bond strength, dentin remineralization, eggshell nano-hydroxyapatite, selective caries removal
Introduction
In modern restorative dentistry, a conservative approach is the prevailing treatment modality for dental caries. The complete removal of caries employed in the treatment of deep lesions is currently regarded as an overtreatment due to the risk of unnecessary and unwarranted removal of sound tooth structure, which can result in a high risk of pulp exposure. Therefore, conservative, selective caries removal is recommended for deep lesions.1 This approach entails the removal of caries from the peripheral and lateral walls of the lesion until sound enamel and dentin margins are reached. For the deepest part of the lesion, the outer softened infected carious dentin is removed, while the inner affected firm layer is left behind. During the process of cavity restoration, demineralized tissue poses a challenge to adhesion when utilized as the bonding substrate, resulting in reduced bond strength values.2 The adhesion to the peripheral sound enamel and dentin is crucial for ensuring the proper sealing of the margins and, consequently, halting the progression of the carious lesion. This is further supported by clinical trials that demonstrated a higher survival rate for teeth restored through the selective caries removal technique.3
As demonstrated in previous literature, the carious process causes a significant reduction in mineral content, especially in deep dentin layers. This is due to a significant increase in porosity and alterations in dentin collagen structure and distribution.4 Consequently, there is a decline in the mechanical properties of dentin, including microhardness, stiffness, microtensile strength, and modulus of elasticity. This reduction affects the quality of the hybrid layer and leads to questionable bond strength and failures under occlusal forces, which has an impact on the overall prognosis of the restoration.5, 6 A biomimetic concept was introduced to regain and restore the normal conditions of the caries-affected dentin substrate and its mineral content through remineralization.7, 8 The application of remineralizing agents prior to bonding has been shown to increase bond strength by restoring the inorganic lost components and reducing the water content and porosity. Consequently, the usage of remineralizing agents on deep caries-affected dentin has the potential to facilitate the recovery of its mechanical properties.9 This approach may be advantageous in cases of subgingival deep cavities where obtaining a sound margin would be challenging.
Different types of dental remineralizing agents were evaluated in literature.10, 11 Sodium fluoride (NaF) solution was first introduced in 1941 by Lulkomsky and is considered the gold standard for desensitization and remineralization.12, 13, 14 In recent years, there has been significant progress in the field of dentistry with the introduction of nano-hydroxyapatite (nHAp), which is expected to be the future of remineralization.15, 16 Nano-hydroxyapatite particles derived from eggshell powder have shown a biomimetic remineralizing effect on enamel surfaces for the treatment of white spot lesions, as well as on exposed dentin for the treatment of erosions.17 Derived from calcium-rich natural sources such as chicken eggshells, nHAp crystals contain a high percentage of calcium carbonate and, to a lesser extent, calcium phosphate, magnesium carbonate and organic matter.18 The high stability and bioactivity of nHAp can potentially provide calcium and phosphate in an adequate concentration to enable repair and promote remineralization. In addition to its antimicrobial properties, a dual action strategy involving mineralization and antimicrobial activity is anticipated from the nHAp.19, 20
Recently, a novel approach has been introduced that utilizes the eggshell-derived nHAp as a remineralizing agent on caries-affected deep dentin substrate. The objective of this approach is to facilitate the restoration of the dentin’s lost inorganic mineral.19, 21, 22 The underlying principle is to achieve a biomimetic tooth restoration complex under functional loading, with the preservation of deep dentin, especially in deep proximal lesions and deep proximal margins that require deep margin elevation. In other studies, the effect of nHAp when incorporated with varying percentages of NaF was examined in an effort to ascertain the optimal remineralization outcome.23, 24
In the domain of adhesion, universal adhesives are regarded as a state-of-the-art development due to their incorporation of 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP), which has been shown to facilitate chemical bonding along with the micromechanical adhesion shown in previous systems.25
A review of the literature regarding the bonding to proximal deep caries-affected dentin, its remineralization prior to deep margin elevation, restoration with composite resin, and its effect on bond strength revealed a paucity of research in this area. Thus, conducting a study to evaluate the microtensile bond strength of resin composite restoration bonded using a universal adhesive to proximal artificially demineralized dentin after treatment with NaF and/or nHAp might be of value.
The purpose of this in vitro study was to examine the microtensile bond strength of resin composite restorations to proximal artificially demineralized dentin and sound dentin in comparison to demineralized dentin following remineralization with 2 agents (NaF solution and eggshell nHAp) either individually or in combination using a universal adhesive system in the total-etch or self-etch mode.
Material and methods
Sample size calculation
The microtensile bond strength was used as the primary outcome, and the mean values were determined based on the results of the study by Barbosa-Martins et al.26 The mean values for microtensile bond strength of 5 groups were 43.32, 21.96, 33.43, 45.25, and 46.42 MPa. The standard deviation (SD) within the group was assumed to be 12 MPa, and the effect size (f) was 0.773. Using an alpha level of 5% and a beta level of 20%, that is, establishing a power of 80%, the minimum estimated sample size was determined to be 6 specimens (rods) per subgroup. The sample size calculation was performed using G*Power v. 3.1.9.2.
Preparation of dentin samples
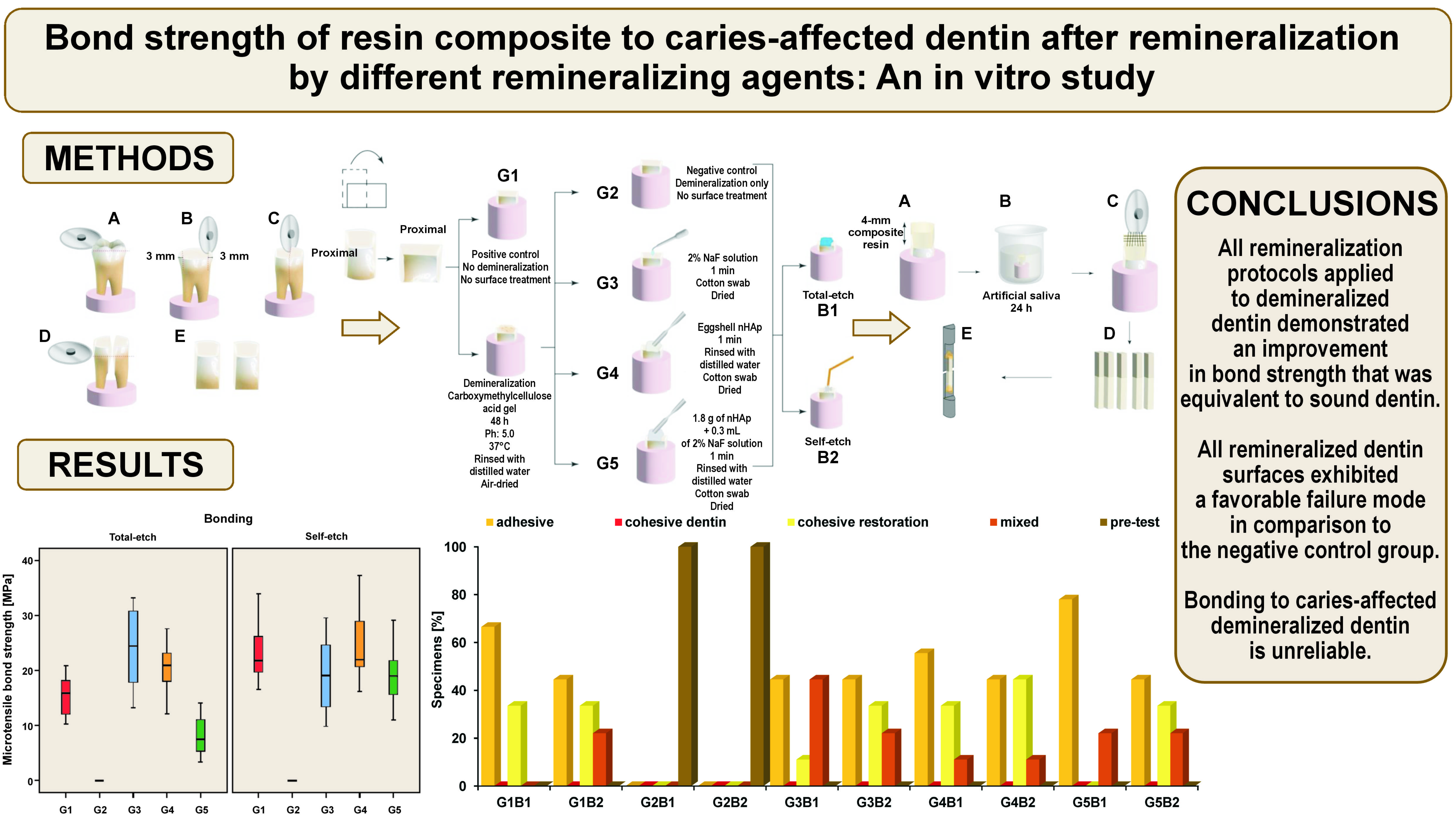
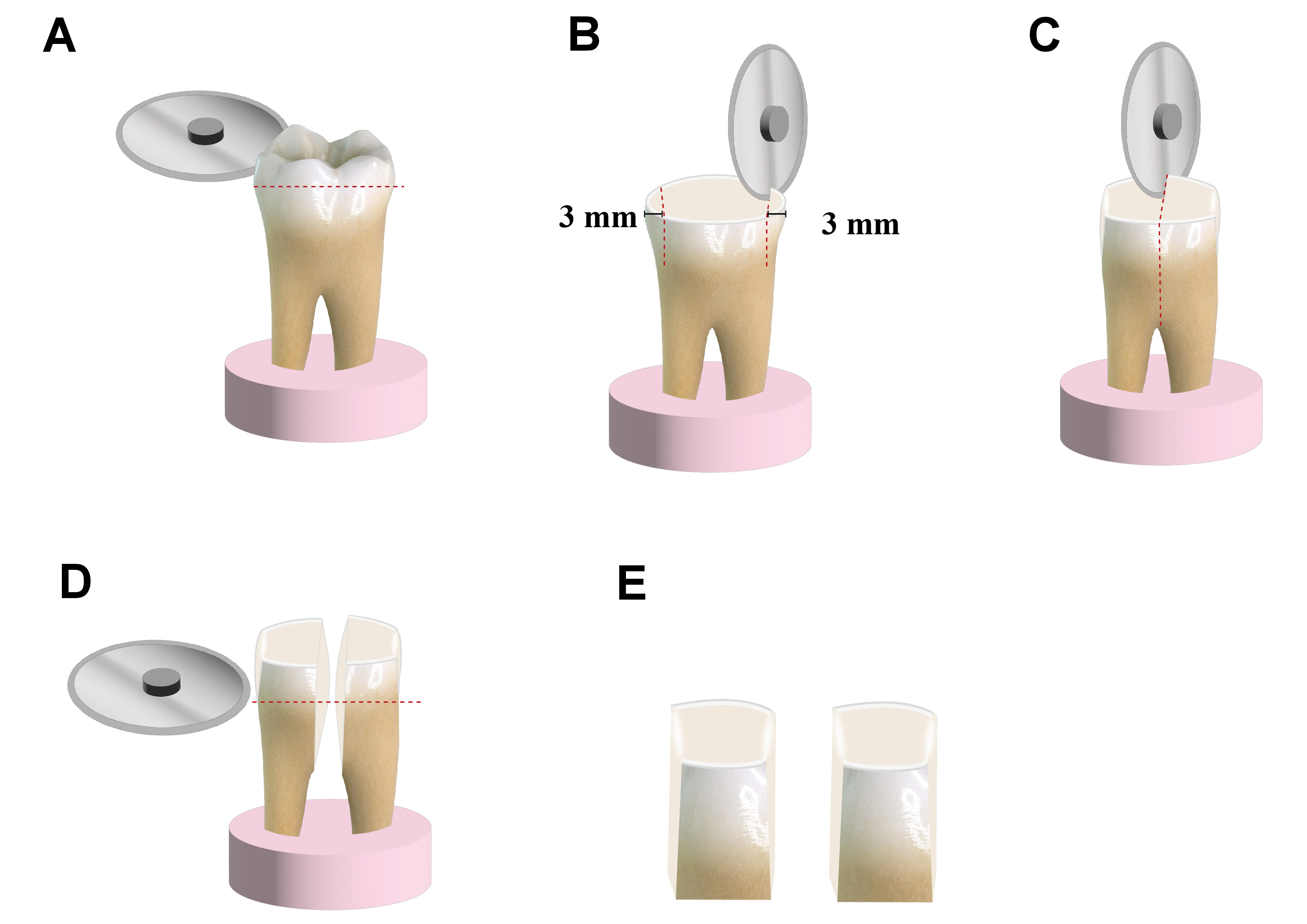
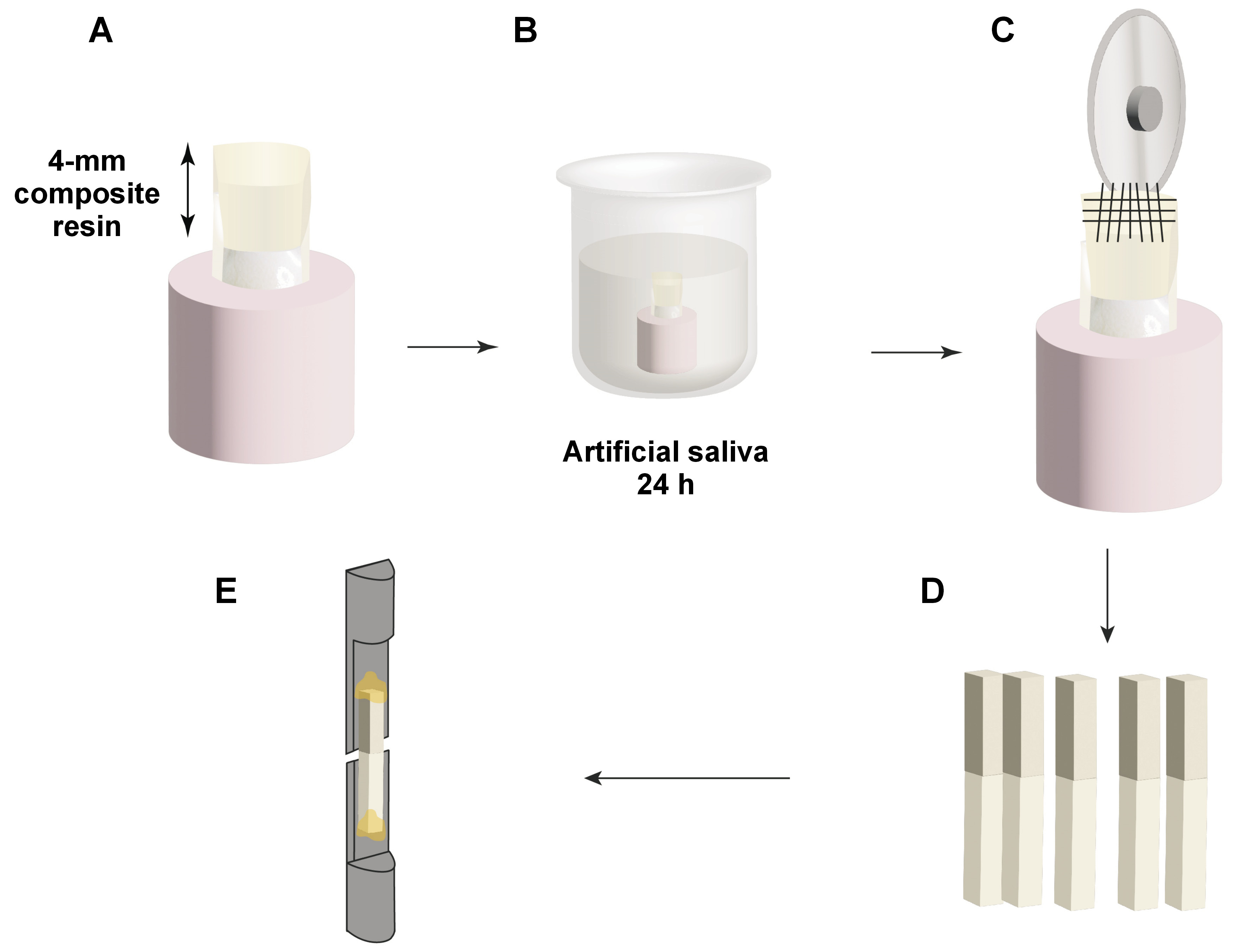
Proximal surfaces of 30 sound molars were used in accordance with the guidelines established by the Academy of Dental Materials (ADM).27 Molars of appropriate size (10 mm or larger mesiodistally) were obtained from the National Institute of Diabetes and Endocrinology, Cairo, Egypt. The teeth were extracted mainly from diabetic patients due to periodontal indications, and the subjects were informed that their teeth would be utilized for research purposes. The teeth were cleaned to remove any soft debris and fixed in acrylic cylinders to allow easy cutting. The occlusal enamel was ground using a water-cooled Sectioning IsoCut® Wafering Blade affixed to an IsoMet 4000 linear saw (Buehler Ltd., Lake Bluff, USA) under copious water coolant (Cool 2 water-soluble anticorrosive cooling lubricant; Buehler Ltd.) to expose the proximal dentin–enamel junction. Subsequently, the proximal surfaces were cut 3 mm from the outer proximal enamel surface, allowing for the examination of deep proximal dentin. In this study, each tooth was utilized as a comparator for the bonding mode. The teeth were decoronated perpendicular to the long axis, the roots were discarded, and the coronal dentin samples were used for the study (Figure 1).
Grouping and randomization
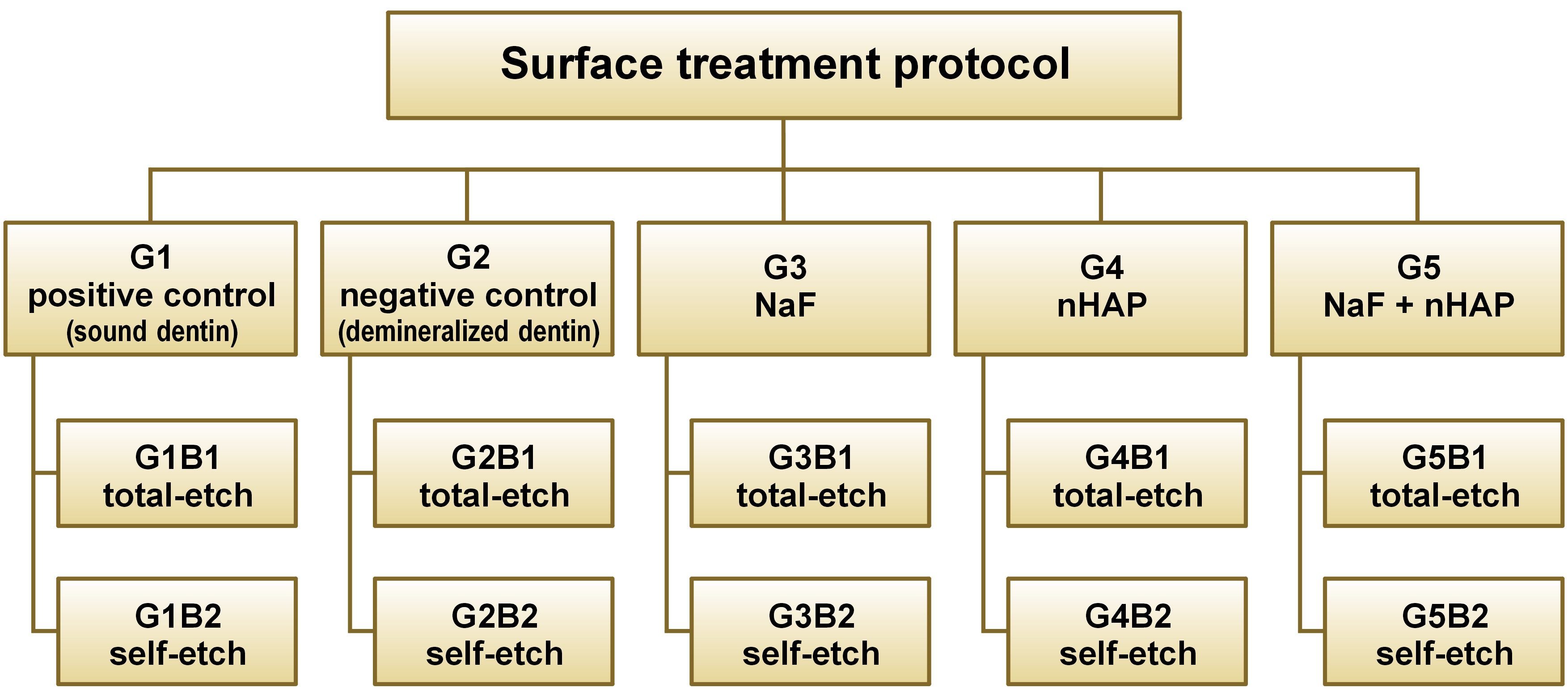
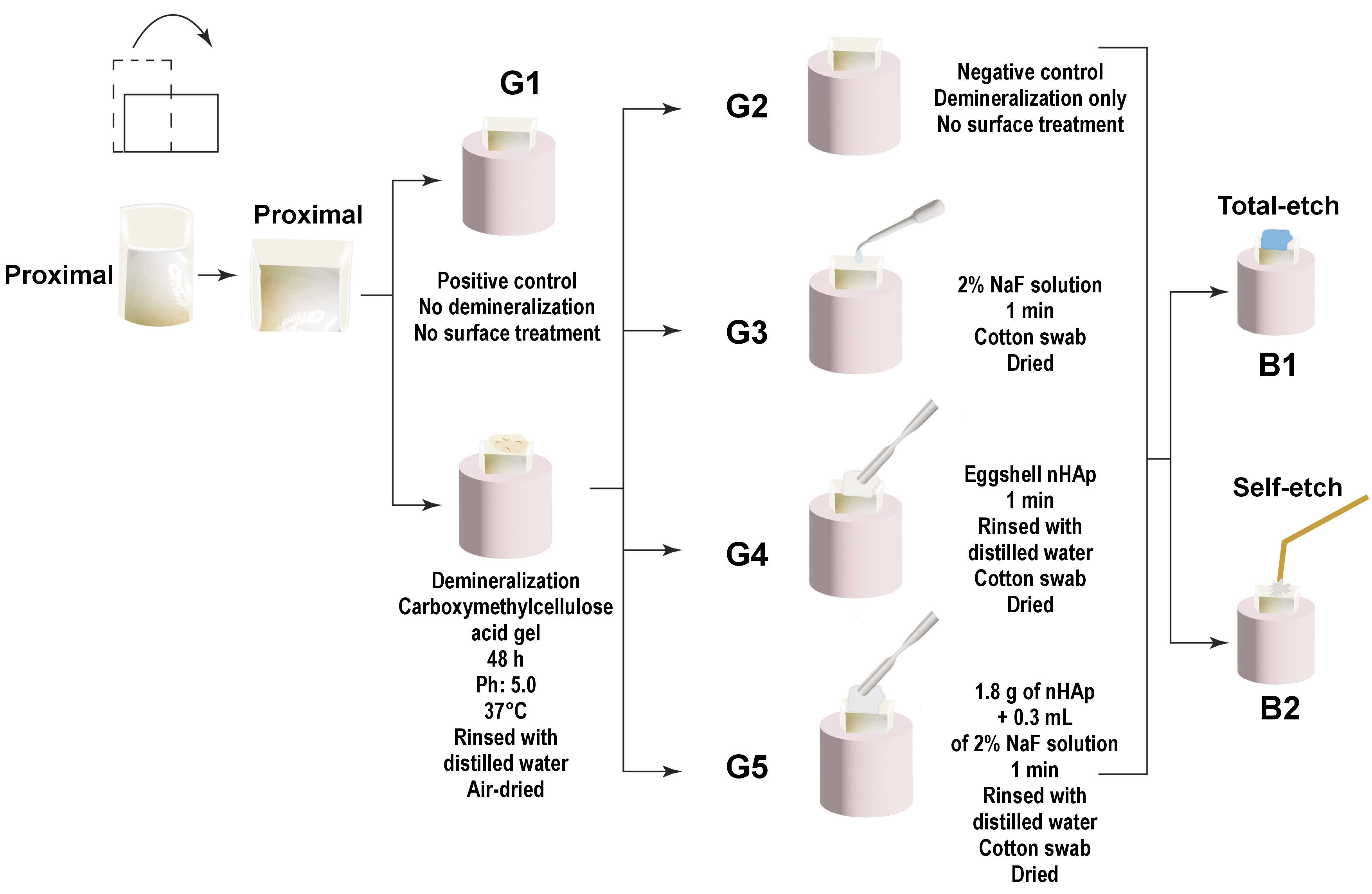
A total of 30 dentin samples (mesial and distal) were fixed in self-cured acrylic resin cylinders. Each sample was coded to ensure that each tooth represented its comparator regarding the bonding mode. Then, the samples were stored in distilled water for 48 h. Subsequently, they were randomly divided into 5 groups according to the remineralization protocols. Group 1 (G1) was the positive control group, in which dentin was left sound without undergoing demineralization or any surface treatment. Group 2 (G2) was the negative control group, in which the dentin surfaces were demineralized and left untreated. In group 3 (G3), the surfaces were demineralized and treated with 2% NaF solution as a remineralizing protocol. In group 4 (G4), the surfaces were demineralized and treated with eggshell nHAp as a remineralizing protocol, and in group 5 (G5), the surfaces were demineralized and treated by a combination of 2% NaF solution and eggshell nHAp. Grouping was performed to ensure that every tooth (mesial and distal halves) received the same remineralization protocol and to overcome the variability in the composition, the heterogeneous nature of dentin, and tooth age.27 Subsequently, each group was subdivided into 2 groups using the previous coding to ensure that each tooth served as its own comparator regarding the application mode of the bonding agent. In this case, B1 represented the total-etch mode, while B2 represented the self-etch mode (Figure 2,Figure 3).
Dentin surface treatment protocols
As illustrated in Figure 3, artificial demineralization was performed in all experimental groups, with the exception of G1. The demineralization process was conducted using a gel composed of 5 mL of 6% carboxymethylcellulose acid gel (0.1 M lactic acid titrated to pH 5.0 in potassium hydroxide (KOH) solution) at 37°C. The deep proximal dentin surfaces were covered in gel for 48 h without renewal. This model has been reported to provide demineralized dentin analogous to that of caries-affected dentin.26 After 48 h, the samples were rinsed with distilled water and air-dried. The following surface treatments were performed in G3, G4 and G5:
– G3: chemically prepared 2% NaF solution (where 5 g of NaF solid (product number 30139; BDH Chemicals Ltd, Poole, UK) was dissolved in 250 mL of water in a polyethylene bottle until complete dissolution, using a magnetic stirrer) was applied using a 1-mm polyethylene plastic pipette. The solution was left on the surfaces for 1 min and blot dried using a cotton swab;
– G4: eggshell nHAp was chemically prepared following the method described by Azis et al.28 The eggshell nHAp powder was dissolved in distilled water (1.8 g of nHAp mixed with 0.3 mL of distilled water), applied using a spatula, and left on the surfaces for 1 min. Thereafter, the surfaces were rinsed with distilled water and blot dried using a cotton swab;
– G5: combination of 1.8 g of eggshell nHAp dissolved in 0.3 mL of 2% NaF solution was applied using a spatula and left on the surfaces for 1 min. Then, it was rinsed with distilled water and blot dried.
Composite resin build-up
In the total-etch groups, the pretreated dentin surfaces were etched with 37% phosphoric acid semi-gel (Meta Etchant; Meta Biomed, Cheongju-si, Republic of Korea) for 15 s, rinsed for 10 s, and blot dried. In the total-etch and self-etch groups, the Single Bond Universal Adhesive (3M™ ESPE, Seefeld, Germany) was applied, rubbed on the surface for 20 s, left to air dry for 5 s, and light cured for 20 s using an LED Light Curing unit (Woodpecker X-Cure; Guilin Woodpecker Medical Instrument Co., Ltd, Guilin, China) with an intensity of 1200 mW/cm2. A Shade A3 (Body) nanocomposite (3M™ Filtek™ Z350 XT; 3M™ ESPE) was used in the fabrication of a 4-mm composite block on the deep dentin surfaces. The application of each 1-mm increment was executed using gold-plated plastic instrument and subjected to light curing for 20 s. The samples were then kept in artificial saliva for 24 h before sectioning (Figure 4).
Microtensile testing
A metal housing with 2 screws was used to firmly attach the acrylic blocks and ensure standard cutting in bucco-lingual and mesio-distal directions (90° to each other). As presented in Figure 4, the 30 samples were transferred to the IsoMet 4000 linear saw (Buehler Ltd.) to allow sectioning of each surface longitudinally in horizontal cuts. Then, vertical cuts were made using a Sectioning IsoCut® Wafering Blade to create rods of similar cross-sectional area (1 mm × 1 mm) and similar remaining dentin thickness. Rods were obtained for each group, with specimens that had pre-test failures excluded from the analysis (n = 9). A total of 9 rods per subgroup and 90 rods for 10 subgroups were expected, yet the negative control group exhibited pre-test failure, resulting in the collection of 72 rods for 8 subgroups. These rods were stored in distilled water. Each beam was then affixed to Gerald Eli’s jig, glued on the jig by its ends using a cyanoacrylate-based glue (705 Universal Fast Adhesive; Akfix, Istanbul, Turkey), a minimum 1 mm from the adhesive interface to allow microtensile testing. The Universal Testing Machine (Model 3345; Instron, High Wycombe, UK) was used to apply microtensile forces to the 72 beams until fracture. Digital software was employed for test control and information acquisition. A tensile load of 500 N was applied to the specimen at a crosshead speed of 0.5 mm/min until the occurrence of bonding failure. The bond strength was calculated in MPa (Bluehill Lite software; Instron, Norwood, USA).
Examination of failure mode
Each fractured beam was examined under a stereomicroscope (MA 100N; Nikon, Tokyo, Japan) at ×30 magnification to ascertain failure modes (Figure 5).
Statistical analysis
The normality of the numerical data was investigated through the examination of its distribution and the implementation of normality tests, namely the Kolmogorov–Smirnov and Shapiro–Wilk tests. The microtensile bond strength data exhibited a non-normal (non-parametric) distribution. The data was presented as median (Me), range, mean (M), and SD values. The Kruskal–Wallis test was used to compare the remineralization protocols. Dunn’s test was used for pairwise comparisons when the Kruskal–Wallis test yielded a significant result. The Mann–Whitney U test was employed to compare between the 2 bonding modes. The failure mode data was presented as frequencies and percentages. Fisher’s exact test was used for the comparisons between the groups. The significance level was set at p ≤ 0.05. The statistical analysis was performed using the IBM SPSS Statistics for Windows software, v. 23.0 (IBM Corp., Armonk, USA).
Results
Comparison of remineralization protocols
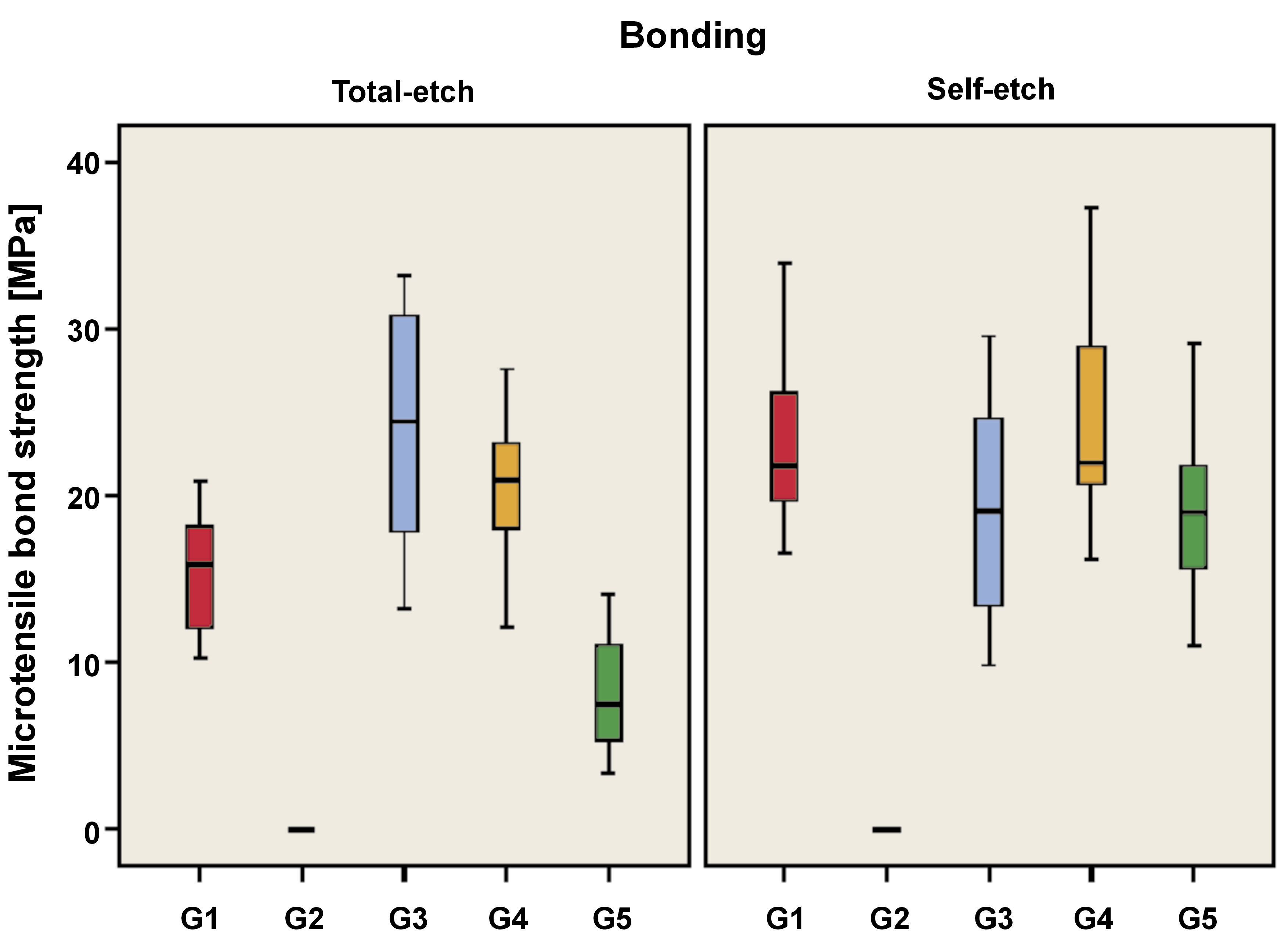
As demonstrated in Table 1 and Figure 6, the utilization of the total-etch mode resulted in a statistically significant difference between the microtensile bond strength values of remineralization protocols (p < 0.001, effect size = 0.789). Pairwise comparisons revealed that there was no statistically significant difference between the NaF and nHAp groups (G3 and G4); both showed the highest statistically significant values. The positive control group (G1) exhibited statistically significant reduction in microtensile bond strength. The combined NaF and nHAp group (G5) demonstrated a similar trend.
The application of the self-etch mode resulted in a statistically significant difference between the microtensile bond strength values of remineralization protocols (p < 0.001, effect size = 0.721). Pairwise comparisons revealed that there was no statistically significant difference between the positive control, NaF, nHAp, and combined NaF and nHAp groups (G1, G3, G4, and G5); all showed the highest statistically significant values. The negative control group (G2) in both bonding modes exhibited the lowest microtensile bond strength.
Comparison of bonding modes
As demonstrated in Table 1 and Figure 6, the total-etch positive control group (G1B1) exhibited statistically significant lower microtensile bond strength values compared to the self-etch positive control group (G1B2) (p = 0.007, effect size = 1.643).
With regard to the negative control, NaF and nHAp groups, there was no statistically significant difference between the microtensile bond strength values of the 2 bonding modes (B1 and B2) (p = 1.000, effect size = 0.000; p = 0.200, effect size = 0.633; p = 0.233, effect size = 0.586, respectively). In the case of the combined NaF and nHAp group, the total-etch group (G5B1) exhibited a statistically significant decrease in microtensile bond strength compared to the self-etch group (G5B2) (p = 0.002, effect size = 2.193).
Failure mode analysis
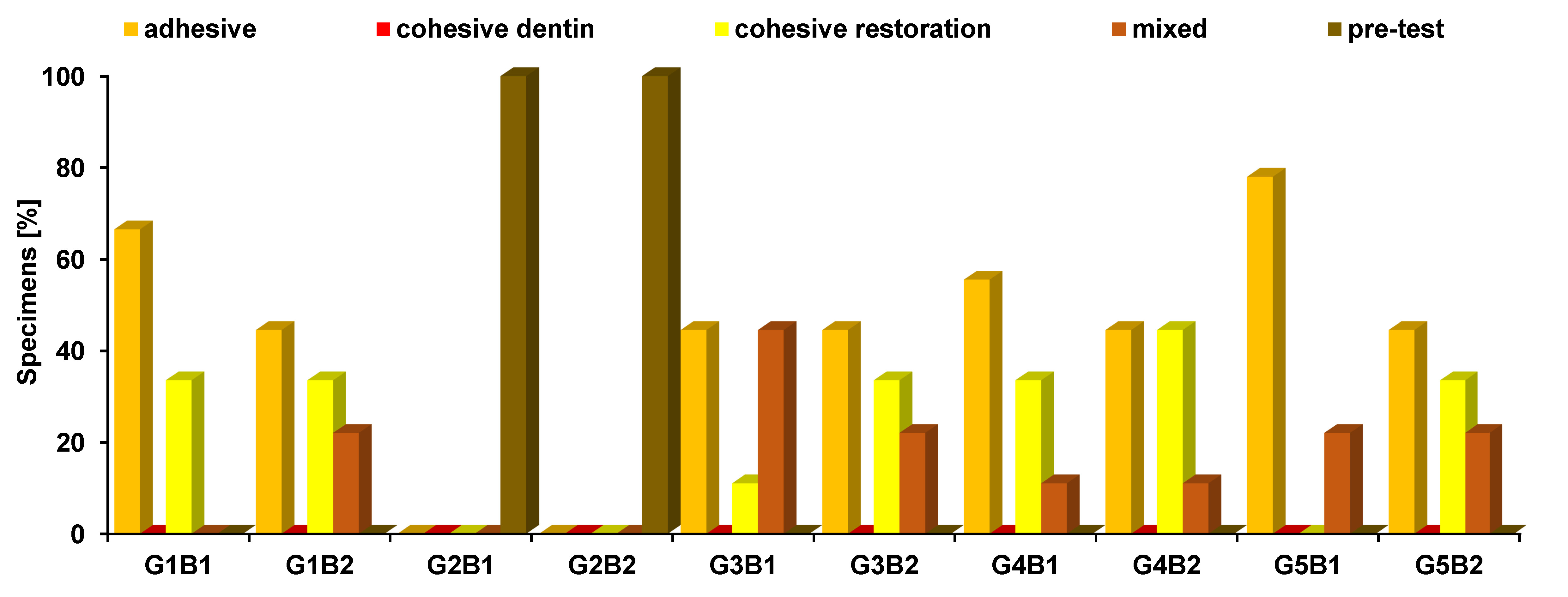
The mode of failure was observed for each beam (n = 9 per subgroup, 9 x 10 = 90, excluding those with pre-test failure, so a total of 72 beams were evaluated) to detect the type of failure as presented in Table 2 and Figure 7. A statistically significant difference was identified between the modes of failure among the different groups (p < 0.001, effect size = 0.615). The G5B1 group demonstrated the highest prevalence of adhesive failure. Groups G2B1 and G2B2 exhibited no instances of adhesive failure. None of the groups showed signs of cohesive dentin failure. The highest prevalence of cohesive restoration failure was observed in the G4B2 group. Groups G2B1, G2B2 and G5B1 demonstrated no cohesive restoration failure. The G3B1 group exhibited the highest prevalence of mixed failure. Groups G1B1, G2B1 and G2B2 showed no instances of mixed failure. All specimens in the G2B1 and G2B2 groups exhibited pre-test failure, and these were the only groups that demonstrated this mode of failure.
Discussion
The complete caries removal approach for deeply cavitated lesions is no longer considered mandatory and is even regarded as an aggressive protocol. In fact, there is growing evidence supporting selective caries removal as the line of treatment for deep carious lesions.29 Theoretically, it is argued that a completely sealed cavity with sound peripheral enamel and dentin margins is necessary for successful bonding and proper restorative procedures, which will allow the remaining carious lesion to arrest. This concept is frequently referred to as “seal is the deal”.30 However, other in vitro studies have shown reduced fracture resistance of incompletely excavated carious tissue and lower bonding strength values of resins to carious dentin.31 Furthermore, a histobacteriological study of human subjects conducted by Ricucci et al. has demonstrated that affected carious dentin contains residual bacteria that may induce subclinical chronic pulpal inflammation when used as a bonding substrate.32
Carious lesions cause a significant decrease in mineral content, especially in deep dentin layers. Concurrently, there is a significant increase in porosity and water content.4 These alterations affect the durability of the bond at the resin–dentin interface, causing a reduction in bond strength over time and an increase in leakage.33 Thus, the adhesion of restorative materials to caries-affected dentin has been proven to result in a bond strength that is lower than that observed for sound dentin, regardless of the adhesive system employed.34 The low bonding efficacy of resin to caries-affected dentin contributes to the deterioration of the adhesive interface of the restoration.5 Previous researchers stated that the clinical management of cases involving caries-affected dentin can be addressed by leveraging the caries-free margins of the restoration, thereby facilitating the bonding to sound enamel and dentin margins.2, 35 However, in cases where deep subgingival carious lesions are present, the attainment of sound cervical margins may not be feasible, necessitating the consideration of surgical crown lengthening, a treatment modality regarded as invasive.36 In addition, other researchers have found that caries-affected dentin left behind may cause biological problems that affect the pulp health and may lead to chronic pulpal inflammation or even silent pulp death.32
As demonstrated in previous studies, the application of a liner prior to restoring a tooth with resin composite improves the quality of deep caries-affected dentin and promotes its self-remineralization.37, 38 Yet, other clinical studies and systematic reviews have shown no difference between the application of a liner and its absence.39 In contrast, this might affect the fracture resistance and overall survival of the restoration due to the absence of direct dentin bonding.40 To overcome these problems and still follow the conservative selective caries removal approach, another concept has evolved. The question has been raised as to whether it would be advisable to remineralize caries-affected deep dentin prior to bonding and restoring the cavity. This concept aimed to overcome the challenges associated with bonding to caries-affected dentin when used as a bonding substrate, improve the quality of the hybrid layer, provide biomimetic protection of the pulp, and enable a conservative treatment for deep class II lesions by facilitating deep margin elevation after remineralization. The remineralization step should be clinically applicable, with ease of manipulation and short application time.
Biological mineralization of dental hard tissues is a progressive dehydration process. As the mineral content increases, the water content of the collagen matrix decreases.7 In dentin bonding, resin adhesive is incapable of dehydrating the collagen matrix sufficiently, leaving behind water that will allow hydrolysis of the hybrid layer components, especially when dealing with deep dentin layers and caries-affected layers.41, 42 Biomimetic remineralization mimics the progressive dehydration of natural biomineralization by replacing matrix water with apatite crystallites.43 In the hybrid layer, replacing water with minerals would increase the mechanical properties and inhibit water-related hydrolysis. In vitro studies indicate that biomimetic remineralization has considerable potential for the restoration of hybrid layers or caries-like dentin, facilitating calcium binding.43 These studies have also demonstrated the preservation of the mechanical properties of dentin, thereby enhancing the quality of hybrid layers.44 The development of clinically applicable materials that contain and release the critical components of the process (at least calcium and phosphate sources) within a clinically applicable time frame poses significant challenges and remains a subject of ongoing research.13
Furthermore, as previously stated, the bonding protocol is considered critical. Therefore, the selection of an appropriate adhesive system and bonding mode may affect the overall outcome. It is anticipated that the total-etch mode may promote the removal of superficial particles that have previously undergone remineralization, thereby impacting the bonding process.35 In contrast, the self-etch adhesive system is expected to improve bonding to the remineralized surfaces, particularly when using universal adhesives, composed of 10-MDP, which has been shown to provide unique bonding not only with micromechanical bonds but also by bonding ionically to calcium and hydroxyapatite in dentin.25, 45
The microtensile test is a method used to evaluate the adhesive properties of resin to dentin. It facilitates the evaluation of regional bond strengths of various portions of the dentin surface.46 The modified testing method employs stick-shaped specimens with a cross-sectional area of 1 mm × 1 mm (non-trimming technique) to enable the testing of large variations in bond strength values that occur due to the formation of serial sections.47 Although microshear could also be used to evaluate the bond strength, it offers a less discriminating evaluation of the adhesive performance. In comparison, microtensile testing provides a more effective means of evaluating dentin adhesion due to its more homogenous stress distribution.48
The current study examined the results of remineralization protocols, revealing that the total-etch groups exhibited a significant difference in the microtensile bond strength values of the NaF and nHAp groups (G3 and G4). Both groups demonstrated the highest microtensile bond strength values, followed by the positive control group (G1) and the combined NaF and nHAp group (G5). The negative control group (G2) showed the lowest microtensile bond strength.
In this study, when NaF (G3) or nHAp (G4) were used in conjunction with the total-etch mode, the bond strength results exhibited a marked increase in comparison to those observed when bonding to sound dentin. This finding aligns with the results of previous studies, which demonstrated that the application of remineralizing agents led to enhanced bond strength values. Okuyama et al. claimed that the application of NaF improves the bond strength to dentin.12 In addition, the findings of Abdallah et al.21 and Roushdy et al.22 suggest that nHAp improves the microtensile bond strength to dentin. However, the findings of these studies did not demonstrate a significantly higher bond strength compared to sound dentin, as observed in the current study. This could be attributed to the correlation between the depth of dentin and an increase in the number and width of dentinal tubules, as well as a decrease in peritubular dentin, which has a high influence on the bond strength. The reduced bond strength is also due to the inadequate mineral ratio present for bonding, particularly when using the total-etch mode with universal adhesives.13 The remineralization of the deep layer, closure of the dentinal tubules and increase in mineral content could promote the bonding when using universal adhesives.
The present study demonstrated that the dissolution of nHAp in NaF in the combined group (G5) resulted in lower microtensile bond strength values when compared to the use of each type separately. This finding contradicts the results of studies by Kunam et al.23 and Rabee et al.,35 which showed higher bond strength when nHAp and NaF were used in conjunction. This phenomenon can be explained as such: the combination of NaF and nHAp results in the formation of fluorohydroxyapatite crystals of increased size, accompanied by enhanced superficial closure and diminished penetration depth. Consequently, it is anticipated that the total-etch mode will facilitate the removal of remineralizing crystals, thereby exhibiting a lesser effect in comparison to the NaF (G3) and nHAp (G4) groups. Furthermore, previous studies applied the remineralizing agents daily for a duration of 7 min. In the current study, a single application for 1 min was employed to enhance its clinical applicability.
In the current study, when the self-etch mode was used, there was no statistically significant difference between the microtensile bond strength values of the positive control, NaF, nHAp and combined groups. This can be attributed to the presence of minerals on the dentin surface, which enhances bonding when using universal adhesives, particularly due to their ionic bonding capabilities to calcium and hydroxyapatite.25 The occurrence of microinterfaces and nano-gaps is expected to be reduced due to the presence of the hydroxyapatite-coated collagen fibrils (nanointeraction zone).49 In the self-etch mode, there was no difference between sound dentin and the different remineralization protocols because the smear layer was not removed completely, and the dentinal tubules were not widely open, in contrast to the total-etch mode.34 For the negative control group (G2), pre-test failures occurred in all the samples, using the total-etch or self-etch protocols. This finding contradicts the conclusions of Nakajima et al., who reported that bonding to caries-affected dentin could be obtained, but with reduced microtensile bonding results and dentin cohesive mode of failure.5
With regard to the different bonding strategies, the current study demonstrated that the positive control group (G1) exhibited significantly lower microtensile bond strength values when the total-etch mode (G1B1) was employed in comparison to the self-etch mode (G1B2). This finding aligns with the study by Pegado et al., which demonstrated that one-step adhesives exhibited superior efficacy in achieving optimal bonding when utilized on deep dentin compared to total-etch adhesives.50 This enhanced bonding capability can be attributed to the presence of functional monomers (such as 10-MDP) in water-based adhesives.50 As previously mentioned, this phenomenon may be due to the increase in the number and width of dentinal tubules and the decrease in peritubular dentin in deep dentin layers. The utilization of the total-etch mode results in the complete removal of the smear layer with a reduction in the mineral ratio necessary for bonding. This alteration could impact the bond strength to deep dentin.13
With regard to the NaF (G3) and nHAp (G4) groups, no statistically significant difference was observed in the microtensile bond strength values of the 2 bonding modes. This outcome could be dependent on the consistency of the remineralizing agent, the size of the particles, and the penetration depth. In the NaF group (G3), the remineralizing agent was administered in the form of a solution (liquid) with high penetration capabilities. In the nHAp group (G4), the agent was applied in the form of a paste with small crystal size (nanocrystals) that exhibited good penetration capabilities. Therefore, the etchant applied in the total-etch mode did not have a detrimental effect on the remineralized surface, and both bonding modes showed analogous results.
In the case of the combined NaF and nHAp group (G5), the total-etch mode (G5B1) exhibited significantly lower microtensile bond strength in comparison to the self-etch mode (G5B2). This phenomenon can be due to the dissolution of nHAp in the NaF solution, which results in the formation of fluorohydroxyapatite crystals of greater size, accompanied by more superficial closure and less penetration depth. Consequently, it is anticipated that the total-etch mode will exhibit a higher degree of remineralizing crystal removal during the etchant step when compared with the self-etch mode.
In the present study, the mode of failure was observed for each beam to identify the type of failure, as it is indicative of the quality of the hybrid layer.43 The highest prevalence of adhesive failure was observed in the combined NaF and nHAp group in the total-etch mode (G5B1), which may be attributed to the high crystal size and shallow penetration, as previously explained. None of the groups exhibited cohesive failure within the dentin, a failure mode commonly reported in the literature when bonding to caries-affected dentin. This finding suggests that the quality of the hybrid layer and the mechanical properties of the caries-affected dentin substrate have been enhanced after remineralization.33, 43, 44 The highest prevalence of cohesive restoration failure was observed in the nHAp group that utilized the self-etch mode (G4B2), indicating that the quality of the hybrid layer has been improved and that a high adhesive bond has been established.21 The highest prevalence of mixed failure was observed in the NaF group that employed the total-etch mode (G3B1). All specimens of demineralized dentin constituting the negative control groups (G2B1 and G2B2) exhibited pre-test failure. These groups were the only ones to demonstrate such a mode of failure, suggesting that the hybrid layer’s quality and the adhesive bond were substandard.
The null hypothesis was rejected based on the results of the current study, which demonstrated a difference in the microtensile bond strength of composite restoration bonded to demineralized and remineralized dentin substrates. The null hypothesis stating that the mode of adhesion (total-etch or self-etch) will not affect the applied remineralizing agent was partially rejected. Additionally, the remineralization step affected the mode of failure.
Limitations
During the preparation of the eggshell nHAp paste, a more diluted consistency might have improved the penetration capacity of the paste and yielded optimal results. Additionally, packable composite resin was applied to the remineralized surfaces without the usage of a flowable composite. The application of a layer of flowable composite resin may have enhanced the adaptability and improved the bond strength. In addition, the application time might have had an influence on the results. The duration of application in the present study was set at 1 min. The impact of varying application times could be further investigated. The variability in deep dentin quality and tooth age may also be a contributing factor, suggesting the necessity for a larger sample size in subsequent studies. The assessment of other dentin mechanical properties after remineralization could also be of value. The findings of this study are limited, as only the immediate effect was evaluated. Long-term studies are recommended to assess the durability of the observed effects.
Clinical recommendations
The findings of the present study indicate that the efficacy of a selective caries removal protocol is contingent upon the presence of sound enamel and dentin margins. In instances where it is not possible to obtain sound margins for the purpose of bonding, such as in cases of deeply seated Class II lesions with subgingival margins, dentin surface treatment with a remineralizing agent prior to bonding may facilitate the recovery of the dentin mechanical properties, thereby rendering them analogous to those of sound dentin. A conservative approach to restorative dentistry involves remineralizing deeply seated caries-affected dentin margins prior to bonding. This method facilitates the elevation of deep margins, particularly in scenarios where obtaining sound margins is challenging.
Conclusions
The utilization of remineralizing agents, such as the eggshell nHAp paste and NaF solution, has been demonstrated to improve bond strength to caries-affected dentin when using universal adhesive in the total-etch and self-etch modes. The combination of NaF and nHAp is only compatible with the self-etch mode, while its use with total-etch adhesives results in a detrimental effect. The bonding ability to caries-affected demineralized dentin is unreliable. The remineralization of caries-affected dentin improves the hybrid layer quality and favors the mode of failure.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board (IRB) of Misr International University, Cairo, Egypt (approval No. MIU-IRB-2122-151).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.