Abstract
Childhood cancer survivors report many health issues related not only to the disease itself but also to post-treatment complications. Dental problems in these patients are irreversible, as they mostly concern the permanent dentition. This systematic review and meta-analysis is aimed at determining the prevalence of hypodontia in cancer survivors. The research strategy was implemented using multiple databases, such as PubMed®, Scopus, Web of Science, and Embase. The literature search was performed on February 21, 2023. A total of 576 articles were screened. Of those, 72 full-text articles were assessed for eligibility, and 31 articles were ultimately selected for inclusion in the meta-analysis. The prevalence of tooth agenesis in pediatric cancer patients was found to be 22% (random effects model; 95% confidence interval (CI): 14–25%, p < 0.001). Pooled analyses of 15 unadjusted relative risk estimates demonstrated a significantly higher prevalence of tooth agenesis in cancer patients compared to healthy individuals (unadjusted odds ratio (OR): 3.12; 95% CI: 2.01–4.83; p < 0.00001). Factors reported in the literature as contributing to the incidence of hypodontia include younger age at diagnosis, the utilization of multiple cytostatic drugs, high-dose radiotherapy (RTX), hematopoietic stem cell transplantation (HSCT), and the presence of other dental abnormalities. Patients who underwent cancer therapy during childhood are more prone to hypodontia.
Keywords: children, cancer, hypodontia, tooth agenesis
Introduction
Childhood cancer survivors suffer from many health problems related not only to the disease itself but also to post-treatment complications. These include cardiometabolic diseases,1 chronic kidney impairments2, 3 and endocrine disorders.4 It is estimated that around 10% of children who survive cancer will experience hearing loss within several years following the disease.5
The curative cancer therapy in children may affect most of the growing and developing tissues, including those of the head and face, such as the teeth. Long-term complications, including hypodontia, microdontia, impaired development of the tooth roots, or demineralization of enamel, may not pose a direct threat to the patient’s life. However, they may adversely affect their health and aesthetics in the future.6, 7 Cancer survivors may also suffer from delayed or accelerated dental development,8, 9 which, in turn, influences the development of the jaws and dental occlusion. The cancer patients were more likely to report at least 1 dental health problem after controlling for socioeconomic factors, age at last follow-up and diagnosis, other treatment exposures, and access to dental services. Consequently, long-term orthodontic or prosthodontic treatment could be necessary.7, 10
The formation of deciduous teeth begins at 4 months of pregnancy, while the first signs of mineralization of the first permanent tooth become apparent at the time of childbirth.11 The cancer treatment can be initiated during the first months or years of the child’s life, when the most active mineralization of permanent tooth buds occurs.12 Therefore, the majority of dental complications become evident later in life of patients with permanent dentition. It has been proven that both chemotherapy (CT) and radiotherapy (RTX) may cause direct or indirect irreversible changes in developing tooth buds. Radiotherapy may directly interfere with the mitotic activity of odontoblasts in developmental patients, resulting in the formation of “osteodentin” rather than the normal dentin and indirectly affecting the process of enamel formation, leading to severe demineralization.6 Cytostatics were also proven to disrupt the metabolic processes and cell cycle of ameloblasts and odontoblasts, thus directly influencing the processes of amelogenesis and dentinogenesis.8, 11
Chemotherapeutic drugs applied in cancer therapy, namely vincristine, doxorubicin, cyclophosphamide, or actinomycin D, exert particularly harmful effects on tooth buds.12 Some cytotoxic antibiotics administered to cancer patients may present relative risks of hypodontia.13 There is evidence demonstrating a relationship between RTX and dental damage, indicating that the dose of RTX correlates with the severity of changes.14 Other studies indicate a relationship between mutations of certain genes and the occurrence of cancer and tooth agenesis.15
Hypodontia, defined as a lower-than-normal number of permanent teeth, results from a complete devastation of tooth buds and is one of the most severe and frequent complications among dental abnormalities experienced by childhood cancer survivors.6, 13, 16 Therefore, the aim of the study was to systematically review the literature to determine the prevalence of hypodontia in pediatric cancer patients and to compare it with the prevalence of the condition in healthy individuals. The null hypothesis stated that the prevalence of tooth agenesis would be comparable in childhood cancer survivors and healthy individuals.
Material and methods
The present systematic review and meta-analysis was conducted according to the PRISMA (Preferred Reporting Items of Systematic Reviews and Meta-Analyses) guidelines in order to follow a uniform and transparent methodology.17 The study was registered with PROSPERO (registration No. CRD42022308068). The following PICOS (Population, Intervention, Comparison, Outcome, and Study design) framework was employed: Population – pediatric patients; Intervention – cancer patients; Comparison – healthy patients; Outcome – prevalence of hypodontia. The research question was: “What is the prevalence of hypodontia in pediatric cancer patients?”
Literature search
The systemic research strategy was implemented using multiple databases, namely PubMed®, Scopus, Web of Science, and Embase. The literature search was performed on February 21, 2023. The search strategy used in PubMed® and adapted in other database searches is presented in Table 1. After the search, all articles were imported into the Mendeley Desktop v. 1.17.11 software (Glyph & Cog, LLC, Petaluma, USA) to eliminate duplicates.
Study selection
The articles were imported into the Rayyan online tool,18 and the titles and abstracts were initially screened to identify studies that potentially met the following eligibility criteria: human experimental studies (cross-sectional and longitudinal, retrospective, and prospective) investigating the prevalence and patterns of tooth agenesis in pediatric patients with cancer; studies with at least 3 subjects with dental anomalies per group. Only manuscripts published in the English language were considered. Case series, case reports, pilot studies, and reviews were excluded from the analysis. The full texts of the articles were reviewed, and a systematic methodology was employed to label all the relevant information for the exclusion or inclusion of individual papers. The decision process was performed by 2 independent reviewers (PP and MLS). In the case of disagreement between the authors, the final decision was made through consultation with a third reviewer (CECS), a senior experienced researcher.
Data extraction
The relevant data from the included studies was extracted independently by 2 authors (PP and MLS) using a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, USA). In instances where information was incomplete or unclear, the authors of the included reports were contacted via e-mail for clarification. The following data was recorded for each included report: study design and sample size; age of participants during examination; age at diagnosis; cancer type; length of therapy; prevalence of hypodontia in cancer patients; and other dental anomalies.
Risk of bias
The risk of bias for all the included clinical trials was assessed by 2 independent reviewers (PP and MLS), and discrepancies were resolved by discussion and in consultation with a third reviewer (CECS). All included studies were evaluated using specific tools for each experimental design: the ROBINS-I (Risk Of Bias In Non-randomized Studies – of Interventions) for non-randomized clinical trials; the Newcastle–Ottawa Scale (NOS) for cohort studies; and the Joanna Briggs Institute (JBI) critical appraisal tool for cross-sectional and case–control studies.17
Statistical analysis
The data regarding the prevalence of tooth agenesis was pooled, and the risk difference with a 95% confidence interval (CI) was used as the effect size. Subsequently, the inverse variance method was selected to calculate the pooled effect. When data from the control patients was available, information regarding the prevalence of tooth agenesis in both cancer and non-cancer patients was used to generate unadjusted odds ratios (ORs) and 95% CIs of the tooth agenesis for the cancer vs. non-cancer group. The heterogeneity (I2) index and Cochran’s Q test were used to examine the heterogeneity between the studies. For the Cochran’s Q test, the p-value was significant at <0.05. All analyses were performed using the MedCalc® statistical software, v. 20.027 (MedCalc Software Ltd, Ostend, Belgium).
Results
Literature search
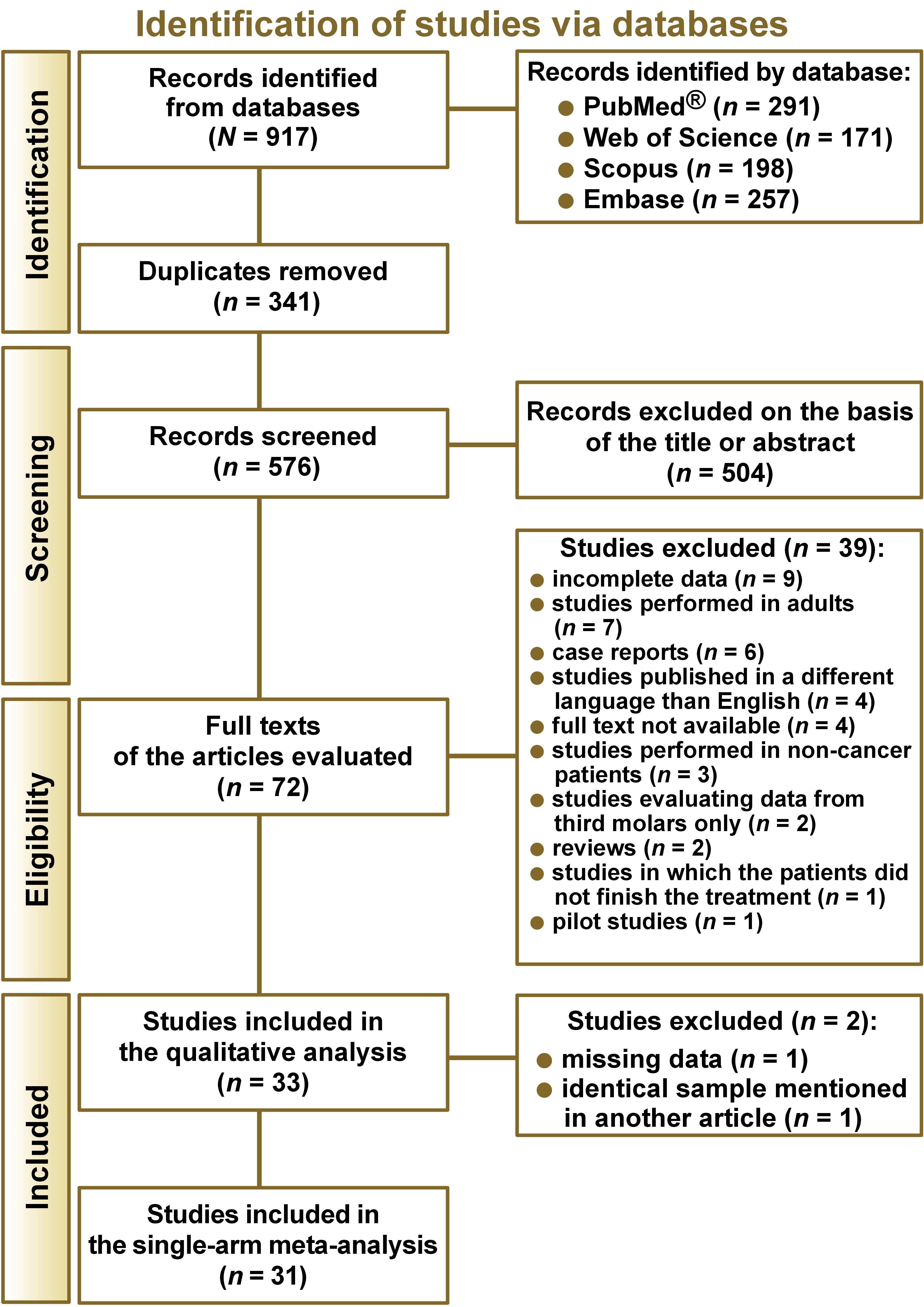
The literature search yielded a total of 917 records (Figure 1). After removing duplicates, 576 articles were screened, resulting in the exclusion of 504 papers based on the eligibility criteria. A total of 72 full-text articles were assessed for eligibility. Of these, 39 were not selected for the qualitative analysis. Nine of them did not present complete data, 7 were based on studies conducted exclusively on adults, 6 were case reports, 4 were published in a language other than English, 4 did not provide the full text, 3 were performed on non-cancer patients only, 2 evaluated data on third molars only, 2 were reviews, one of the studies included patients who did not complete the treatment, and 1 was a pilot study. A total of 33 studies were included in the qualitative analysis. However, 2 additional articles were excluded: one due to missing data; and the second one because it employed the same sample as another article. Finally, 31 studies were included in the single-arm meta-analysis.3, 6, 8, 9, 16, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 Only 14 studies presented data for a control group and were included in the proportion meta-analysis.6, 12, 19, 20, 21, 22, 29, 30, 31, 32, 33, 34, 36, 44, 45
The characteristics of the included articles are summarized in Table 2. Several types of clinical studies were included, such as cross-sectional, cohort and case–control studies. In the investigated groups, the number of cancer patients ranged from 10 to 9,308. The subjects suffered from various forms of cancer, including solid tumors, leukemias and lymphomas. They were most often treated with CT alone; however, some patients also received RTX (including head and neck RTX), total body irradiation (TBI) and hematopoietic stem cell transplantation (HSCT). At the time of diagnosis, the majority of patients were under 10 years of age, with the youngest subject being 1 month old.
Various teeth were affected by agenesis. Most often missing teeth were second premolars, second molars and lower incisors.6, 8, 16, 19, 21, 22, 28, 30, 35, 36, 37, 43 Risk factors associated with a higher incidence of agenesis in cancer survivors were: younger age at diagnosis or treatment (1–7 years)3, 8, 16, 19, 20, 23, 27, 29, 32, 34, 35, 36, 37, 40, 43, 44, 46, 47, 48; use of multiple (>4) classes of chemotherapeutic agents, particularly alkylating agents in high doses, and prolonged duration of therapy3, 16, 30, 37, 38; use of heavy metal compounds in CT2; RTX dosage greater than or equal to 2,200 cGy32, 44; head and neck radiation therapy (RTX)23, 35, 40; history of HSCT3, 37, 38, 43; and the presence of other dental anomalies.16
Risk of bias
For cross-sectional studies, the average quality score ranged between 4 and 6 (Table 3). The criteria that exhibited the highest failure rate pertained to the identification of confounding factors. For cohort studies, the quality score ranged between 4 and 6 (Table 4). The studies under review failed to complete the independent blind assessment. Non-randomized clinical trials were catalogued as having a high risk of bias in domains of confounding and selection of participants into the study (Table 5). For case–control studies, the quality score ranged from 5 to 8. However, all studies failed to meet the criteria related to the identification and management of confounding factors (Table 6).
Meta-analysis
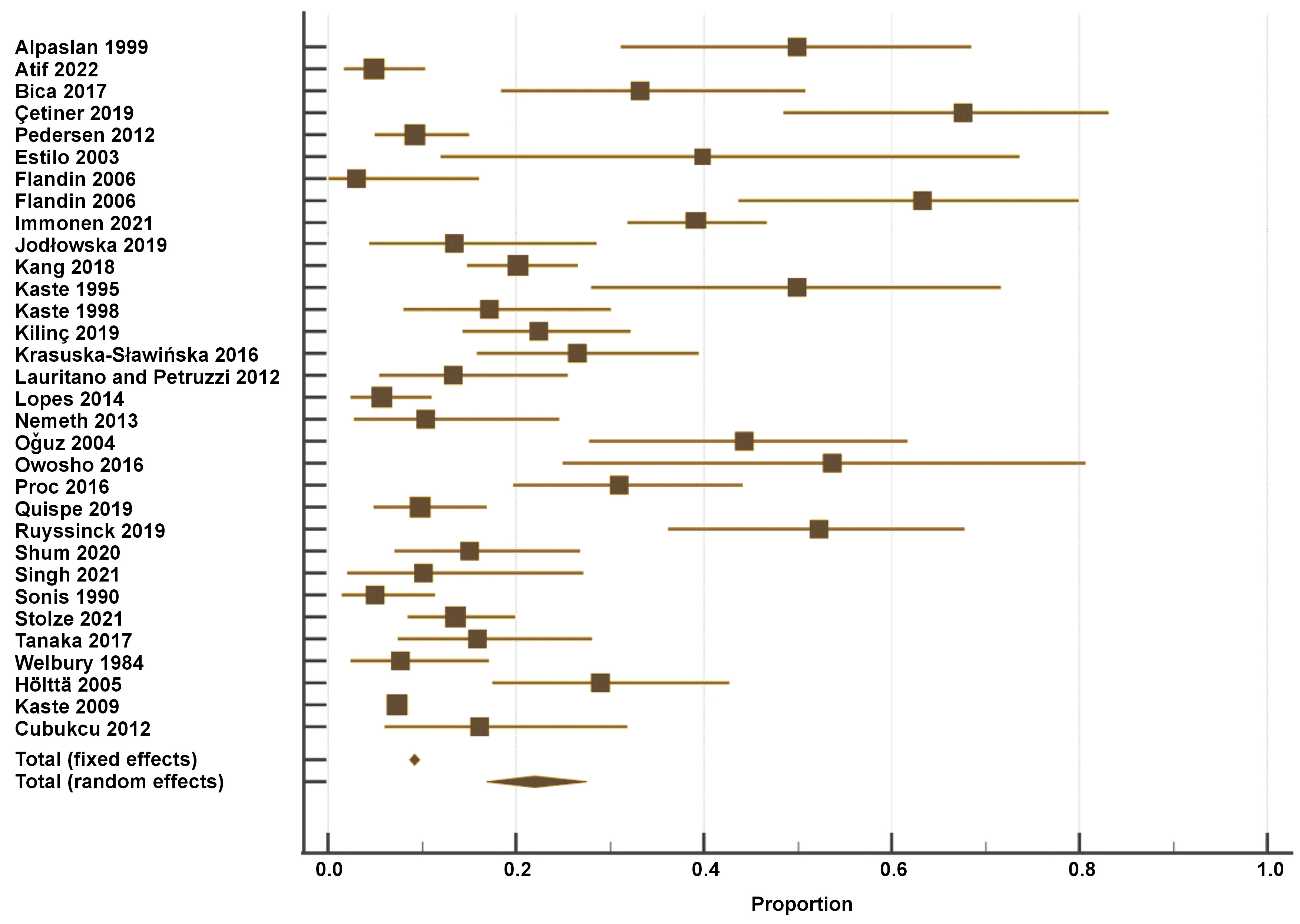
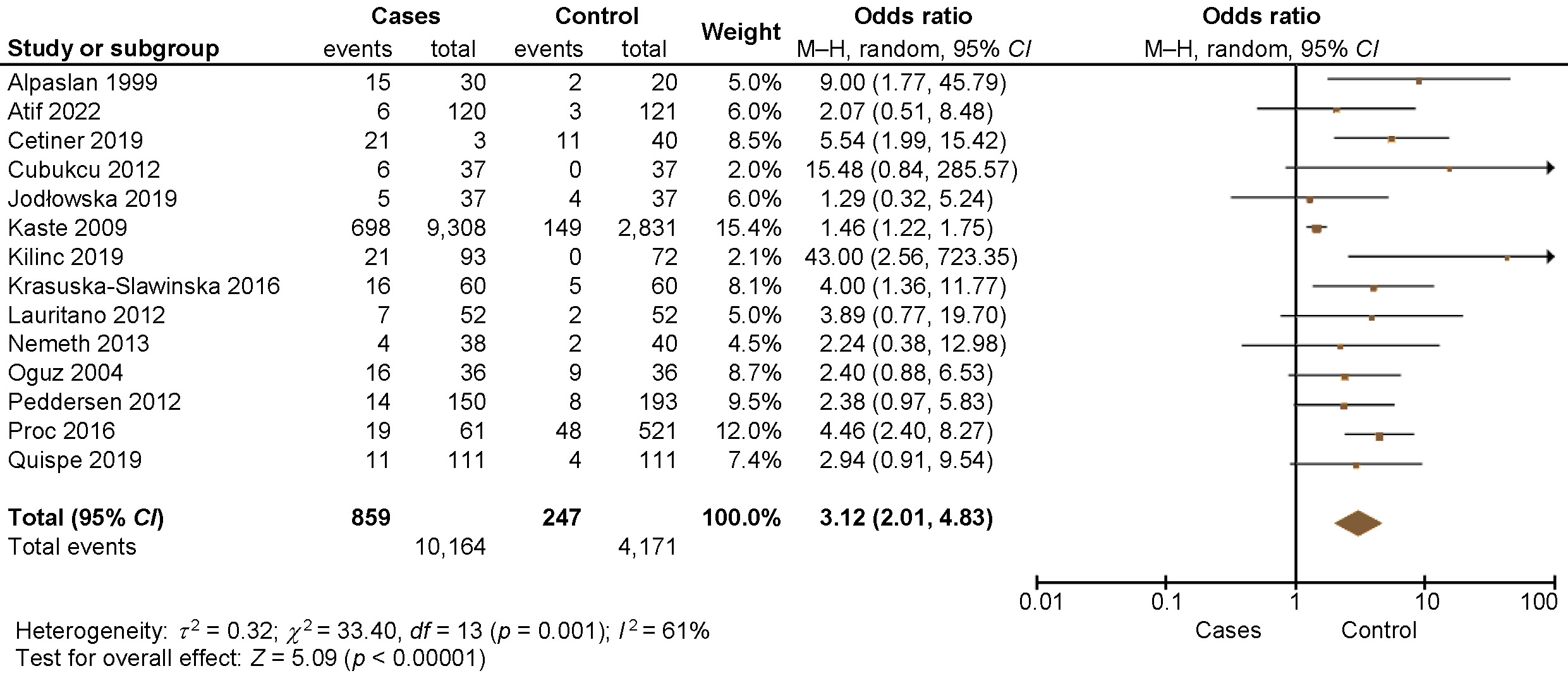
Figure 2 presents the results of the single-arm meta-analysis, which revealed that the prevalence of tooth agenesis in pediatric cancer patients was 22% (random effects model; 95% CI: 14–25%, p < 0.001). Pooled analyses of 15 unadjusted relative risk estimates demonstrated a statistically significant 2.94-fold increase in the prevalence of tooth agenesis in cancer patients compared to non-cancer patients (unadjusted OR: 3.12; 95% CI: 2.01–4.83; p < 0.00001) (Figure 3). Dental abnormalities were found to be more common among cancer patients than in healthy controls in most of the reviewed studies.6, 19, 21, 30, 31, 33, 44, 45 All the details are provided in Table 2.
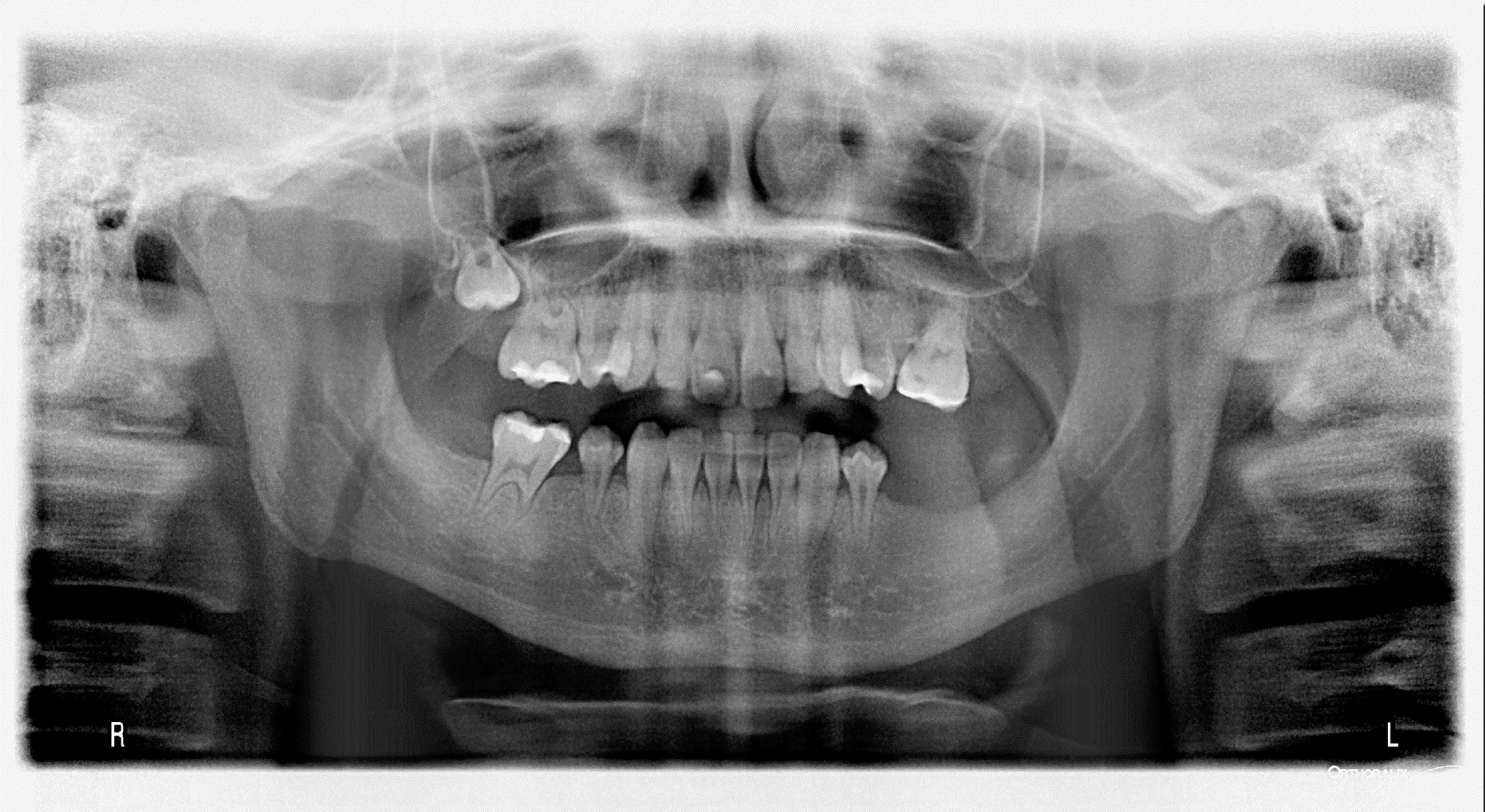
Figure 4 presents an exemplatory panoramic radiograph of a 15-year-old male patient diagnosed with neuroblastoma at the age of 3. The patient underwent a 21-month treatment regimen consisting of combination therapy, which included high-dose and conventional CT, bone marrow transplantation and RTX. The patient suffers from hypodontia, short roots of teeth and microdontia.
Discussion
This systematic review was aimed at assessing the prevalence of tooth agenesis in childhood cancer survivors and healthy individuals. The findings revealed that the occurence of hypodontia was higher in children who had undergone cancer treatment compared to their healthy peers. The null hypothesis stating that childhood cancer survivors and healthy individuals would have the same prevalence of tooth agenesis was rejected. The presence of defects depended on various factors, including both individual characteristics of the child and the applied treatment. According to the peer-reviewed articles, hypodontia was estimated to affect between 1.4% and 66.42% of cancer patients.3, 6, 8, 16, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 In the healthy group, the prevalence of hypodontia ranged from 0% to 25%.29, 34 The number of missing teeth in the cancer groups ranged from 6 to 69.6, 19, 27, 33, 35, 36, 46 It was also found that 15–85% of third molars were missing in cancer patients.19, 21
The teeth most frequently affected by agenesis were second premolars and second molars. In healthy individuals, the most often missing teeth were lateral incisors. The prevalence of specific groups of microdontic teeth depended on the time of the treatment and the conditions of the most active mineralization.13 A similar trend was observed with respect to the prevalence of hypodontia in particular tooth groups; however, the difference was not statistically significant. This phenomenon can be explained by the observation that, when exposed to particularly strong external factors, tooth buds undergo complete degradation, irrespective of their development stage.
Moreover, the overall dental development, as expressed by dental age, varied in cancer survivors and depended on the type of cancer and the implemented therapy.5 In the majority of cases, the dental age of cancer survivors was accelerated, predominantly due to premature closure of root apices. The dental age was significantly delayed in patients with familial adenomatous polyposis (FAP)-associated hepatoblastoma. However, the changes in dental age were independent of sex, age, or the duration of treatment.9, 49
Numerous factors can influence the occurrence of hypodontia, with the most prevalent one being the age of the patient at the time of diagnosis and therapy. The younger the age of the child at the time of diagnosis, the earlier the stage of tooth development and the greater the risk of more serious dental defects. The significant age limit varied in different publications, although it was consistently below 7 years of age.3, 8, 16, 19, 20, 23, 27, 29, 32, 33, 34, 35, 36, 37, 40, 43, 44, 46, 47 This is in line with the time of the most active development of tooth buds, which is considered to be the age between 6 and 8 years.6
The age at diagnosis correlated not only with the frequency but also with the severity of dental abnormalities. The patients in the youngest group presented with tooth agenesis or microdontia, while those in the oldest group demonstrated the most prevalent occurrence of abnormal root development.6, 20, 23, 26, 27, 29, 34 Additionally, the prevalence of combined disturbances was significantly lower in the youngest group compared to the other groups.2 The co-occurrence of different dental defects was frequently observed, as most of the cancer survivors suffered from more than one type of abnormality.5 Apart from hypodontia, the most frequently reported complications were microdontia, root deformation with premature apexification, enamel discoloration, and unerupted teeth. Patients with rhabdomyosarcoma of the head or neck who underwent treatment, including RTX, suffered from oral diseases, i.e., bony hypoplasia/facial asymmetry, trismus, velopharyngeal insufficiency, radiographically underdeveloped mandible, severe malocclusion, caries, hyposalivation/xerostomia, and gingivitis.23, 27, 35 On the other hand, factors like malocclusion, trauma, severe pain stimuli, parafunctional activities, and psychological elements, including stress, anxiety and depression can lead to temporomandibular disorders (TMD).50
It is worth noting that the dose, type and number of cytostatic drugs administered were identified as risk factors for hypodontia and other dental defects. The use of more than 4 different chemotherapeutic agents and heavy metals has been identified as a significant risk factor for severe dental disturbances.2 Additionally, chemotherapeutic drugs such as vincristine, cyclophosphamide, doxorubicin, ifosfamide, etoposide, and cisplatin significantly increased the risk of tooth agenesis.5 Interestingly, it has been reported that equivalent doses of cyclophosphamide above 8,000 mg/m2 are associated with a higher number of teeth missing due to agenesis.6
Total body irradiation is performed in cancer patients to suppress the immune system and prevent the rejection of bone marrow transplantation (BMT).7 The side effects of TBI are most pronounced in terms of height and weight delay, while other complications of TBI include hypothyroidism, cataracts and a high incidence of secondary tumors.6 However, dental complications, such as tooth agenesis, were not found more frequently in the group of patients who had undergone TBI treatment.24, 43 As for patients treated with TBI, agenesis was more frequent in individuals receiving busulfan (63.2%) than in those treated with other chemotherapeutic agents (37.5%).7
On the other hand, some studies have documented a significantly higher prevalence of tooth agenesis in children treated with HSCT (similarly to BMT).2 The prevalence of agenesis and microdontia affecting at least 1 permanent tooth in cancer patients who had undergone HSCT treatment was much higher when compared to the controls. Moreover, 92.3% of children aged ≤3 years old at the time of HSCT treatment exhibited tooth agenesis.5 The condition manifested more prominently in certain tooth groups, including first and second premolars in the maxilla and mandible, as well as second molars in the mandible (all p-values <0.001).6
The relationship between the application of head and neck RTX and the occurrence of dental changes was also investigated.23, 35, 40 The radiation exposure of ≥20 Gy to the dentition was significantly associated with an increased risk of 1 or more dental abnormalities.6 After RTX, the frequency of dental changes reached from 80% up to 100% among children under 5 years of age.23, 40
Impaired tooth development constitutes a complication that arises subsequent to cancer treatment. Tumor-induced osteomalacia has been widely described in patients ranging in age from 9 months to 90 years, with a broad spectrum of tumor types. In adults, the primary concern is a decreased level of serum phosphate, while in children (aged <18 years), it is a low or improperly circulating concentration of 1,25-dihydroxyvitamin D.51 The 1,25-dihydroxyvitamin D, in turn, belongs to the group of interacting circulating hormones and their key receptors that regulate the state of calcium homeostasis.52 Calcium and phosphate play a key role in the mineralization of teeth and bones. Disturbances in the levels of these minerals during the developmental phase of an organism may partially account for the increased occurrence of dental defects in childhood cancer survivors.
Tooth agenesis is more prevalent among cancer survivors in comparison to healthy controls. There are several factors related to cancer and its treatment that contribute to the occurrence of agenesis. Given the high risk of complications in cancer patients, increased dental attention and care are required.
Conclusions
Patients who underwent childhood cancer treatment may experience dental complications more frequently compared to the general population. The dissemination of knowledge on this subject among clinicians is necessary to ensure the provision of specialized dental care to such patients, thereby facilitating their recovery and enhancing their quality of life.
Trial registration
The study was registered with PROSPERO (registration No. CRD42022308068).
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.