Abstract
Background. These days, computer-aided design/computer-aided manufacturing (CAD/CAM) resin-based materials are being used daily in clinical practice for restorations in prosthetic dentistry. In comparison with ceramic materials, they are characterized by better stress distribution and decreased abrasion of the enamel of the opposing teeth. Consequently, they have been applied as alternative materials to ceramics in various dental restorations.
Objectives. The contamination of the indirect restorative material, which occurs at the clinical and dental laboratory stages, might deteriorate the bonding strength. The ideal surface treatment of the novel polyetheretherketone (PEEK) material for decontamination is yet unknown. The present study was conducted to evaluate the tensile bond strength (TBS) between PEEK and dual-cure self-adhesive resin cement, and determine the effect of contaminants, like temporary cement, artificial saliva and a fit checker, as well as the cleaning methods, like ultrasonic cleaning, phosphoric acid etching and universal cleaning paste (Ivoclean), on the bond.
Material and methods. Eighty PEEK disks were milled, having the final dimensions of 12 mm × 4 mm. The specimens were air-abraded with 50-micrometer aluminum oxide particles at a pressure of 2.8 bar for 15 s at a fixed distance of 10 mm, and then divided into 4 groups according to the contaminant used: temporary cement; artificial saliva; a fit checker; and a control group with no contamination. Furthermore, the first 3 groups were subsequently subdivided into 3 subgroups each according to the cleaning method applied: ultrasonic cleaning; phosphoric acid etching; and universal cleaning paste Ivoclean. The bonding of the specimens was done using dual-cure self-adhesive resin cement. The TBS of the different groups and subgroups was then measured at a crosshead speed of 2 mm/min in a universal testing machine (UTM), using a special test configuration.
Results. There was a significant interaction between both tested variables (the contamination and cleaning methods) (p < 0.001). The samples contaminated with artificial saliva showed a significantly higher TBS value than the samples subjected to other contaminants (p = 0.005). For the samples contaminated with temporary cement and a fit checker, there were significant differences between the different cleaning methods, with ultrasonic cleaning providing the highest TBS values, followed by phosphoric acid etching, and finally Ivoclean (p < 0.001).
Conclusions. Under the conditions of the present study, temporary cement and a fit checker adversely affected the TBS of PEEK, and ultrasonic cleaning was most effective for decontamination.
Keywords: contamination, PEEK, bonding, cleaning, tensile bond strength
Introduction
These days, computer-aided design/computer-aided manufacturing (CAD/CAM) resin-based materials are being used daily in clinical practice for restorations in prosthetic dentistry.1 In comparison with ceramic materials, they are characterized by better stress distribution and decreased abrasion of the enamel of the opposing teeth. Consequently, they have been applied as alternative materials to ceramics in various dental restorations.2
The novel high-performance composite polyetheretherketone (PEEK) is a polymer derived from the main group of polyaryletherketones (PAEKs), initially used in orthopedic and spinal implants. It is considered a biocompatible material, and it is chemically stable with regard to nearly all organic and inorganic chemicals.3, 4 With a stiffness comparable to that of bone, PEEK has been used for fixation plates.5 It is mainly composed of an aromatic backbone molecular chain with ketone and ether functional groups interconnected to it.
The earlier literature on PEEK has shown that it has better chemical, thermal, biological, and mechanical properties – a high strength-to-weight ratio, a modulus of elasticity similar to that of bone and dentin, in addition to its zero corrosion rate and extremely low water absorption – in comparison to many restorative materials used today.6
Polyetheretherketone is used in dentistry for implants, provisional abutments and implant-supported bars. Recently, it has been found suitable for fixed dental prostheses (FDP) and resin-bonded fixed dental prostheses (RBFDP) as an interim treatment option, due to its high resiliency and a low modulus of elasticity. These properties may reduce stress concentration at the cementation interface and prevent debonding.7 Due to its low translucency and white opaque color, PEEK is not suitable for monolithic, esthetic dental restorations, and a composite veneering material or cemented ceramic crowns over PEEK frameworks are required to achieve satisfactory esthetics.8 Also, PEEK has low surface energy, and many studies have analyzed different surface conditioning methods to increase its surface free energy, and thus optimize its bond strength.9, 10, 11 Kern and Lehmann, studying the influence of surface conditioning on bonding to PEEK, demonstrated that after air abrasion and applying a resin varnish with a methacrylate group, promising and durable bonding was achieved.12 Uhrenbacher et al. also stated that the type of conditioning had a significant effect on the retention strength of PEEK.13 A clinical implication of their study is that airborne-particle abrasion (with 50-micrometer alumina) or etching with sulfuric acid (for 60 s) and methyl methacrylate (MMA)-based adhesive systems can be used for conditioning. In a review by Skirbutis et al., regarding articles published between 2010 and 2017 on PEEK and its characteristics, it was concluded that the highest bonding values were obtained by conditioning with airborne-particle abrasion and sulfuric acid surface treatment, as well as for crowns pretreated with the Signum® universal bond system and the visio.link adhesive system.9
Contamination reduces the adaptation between the restorative material and the bonded surface, thus inhibiting the formation of a long-term, stable bond.14, 15, 16 Therefore, the cleaning of both surfaces, or either the substrate or the material, is essential to improve the strength of the bond and its durability.16 During the try-in procedure, bonding to ceramics, zirconia and resins can be compromised by contamination. Saliva, blood, a silicone indicator, and die stone have been identified to reduce the bond strength of resins to restorations.17, 18 Since the efficiency of the cleaning methods in removing contaminants from the adherend surface varies by method and material, it is critical for the clinician to use the most effective cleaning method for the material used prior to bonding to achieve the best bond strength of the restoration. Studies have determined the effect of different cleaning protocols on zirconia and ceramics.19, 20
Polyetheretherketone has significant advantages to be used for dental applications. However, a major clinical disadvantage is the difficulty in establishing strong and durable adhesion to other dental materials. Since there are 2 bonded interfaces with resin cement – the tooth structure and the veneering esthetic crown material – current studies focus on enhancing the PEEK surface for reacting with resins to allow optimal bonding.21 Bonding to PEEK is considered a challenge due to the contaminants present in the oral cavity, like saliva, temporary cement or a fit checker, and also its low surface energy, poor wetting capabilities and resistance to surface modification.
Debonding is one of the major failures in prosthetic dentistry, especially for PEEK with its low surface energy, which causes problems regarding surface treatment and conditioning.9 For that reason, this study was conducted to evaluate the tensile bond strength (TBS) between PEEK and dual-cure self-adhesive resin cement, and determine the effect of contaminants, like temporary cement, artificial saliva and a fit checker, as well as the cleaning methods, like ultrasonic cleaning, phosphoric acid etching and universal cleaning paste (Ivoclean), on the bond. The null hypothesis was that there would be no effect of the contaminants and the cleaning methods on the TBS between PEEK and the resin cement.
Material and methods
Power analysis
A power analysis, using G*Power, v. 3.1.9.7 (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower), was performed in order to calculate the smallest sample size necessary to test the null hypothesis: no difference in TBS between the tested groups. The sample size (N) was established at 60 samples (i.e., 6 samples per subgroup), with an alpha level α = 0.05, a beta level β = 0.2 (i.e., a power of 80%) and an effect size f = 0.554. The calculation was based on the findings from a previous study.22 The sample size was raised to 80 samples (i.e., 8 samples per subgroup) to accommodate for the anticipated testing failures.
Preparation of specimens
A total of 80 milled PEEK disks (KERA® starPEEK; Eisenbacher Dentalwaren ED, Wörth am Main, Germany), with dimensions of 12 mm in diameter and 4 mm in thickness, were used in this study. The disks were divided into 4 main groups according to the contaminant used: group T (n = 24) – temporary cement (RelyX™ Temp NE, 3M ESPE, Maplewood, USA); group S (n = 24) – artificial saliva (prepared at the Laboratory of Biochemistry at Ain Shams University, Cairo, Egypt, using 1 L of double-distilled H2O, 1.68020 g NaHCO3, 0.41397 g NaH2PO4·H2O, and 0.11099 g CaCl2); group F (n = 24) – a fit checker (Fit Checker; GC International, Tokyo, Japan); and group C (n = 8) – the control group without contamination. The first 3 groups were further divided into 3 subgroups (n = 8) according to the cleaning method applied: subgroup U – ultrasonic cleaning (ultrasonic cleaner CD 4862; Codyson, Shenzhen, China); subgroup P – phosphoric acid etching (N-Etch; Ivoclar Vivadent, Shaan, Liechtenstein); and subgroup I – universal cleaning paste Ivoclean (Ivoclar Vivadent), which is composed of zirconium oxide (10–15 wt%), water (65–80 wt%), polyethelene glycol (8–10 wt%), and sodium hydroxide (≤1 wt%).
All study procedures were carried out by the same operator and according to the manufacturers’ recommendations. The milled 80 PEEK disks were air-abraded with 50-micrometer aluminum oxide particles at a pressure of 2.8 bar for 15 s at a fixed distance of 10 mm, and then cleaned in an ultrasonic bath of distilled water for 3 min to remove any residues from their surfaces. Afterward, the specimens were air-dried with compressed air for 15 s to remove any remaining surface liquid.
Contamination
For group T, the temporary cement was mixed on a glass slab in a 1:1 ratio and applied with a spatula to the surface of each disk. After placing a glass slab on the cement, pressure was applied to it. To standardize the thickness of the cement layer and allow enough time for the cement to set, a load of 2 kg was applied for 5 min, using a cementation device, specially designed for applying pressure during the cementation process. The disks contaminated with the temporary cement were stored in water at 37°C for 24 h. For group S, artificial saliva was applied, rubbed for 60 s with a micro-brush into the surface of each disk, and then left undisturbed for 10 s. For group F, a fit checker was applied using an Automix gun and Automix tips on the surface of each disk, which were then stored in water at 37°C for 5 min.
Cleaning
The contaminants were removed from the disk surfaces of the 3 groups. Group S specimens were air-water-sprayed for 20 s, and then dried. The contaminants in groups T and F were removed with the tip of a blunt instrument. Each of these groups was further divided into 3 subgroups according to the cleaning method. For subgroups U, 8 disks from each contaminant group were immersed in an ultrasonic cleaner filled with 99% isopropanol alcohol, and left for 3 min. Then, each disk was air-dried for 15 s. For subgroups P, 8 disks from each contaminant group were cleaned using 35% phosphoric acid. Phosphoric acid was applied to the surfaces of the specimens with a tube tip and left on the surface for 1 min. Each disk was then cleaned with a water spray for 15 s and air-dried for 15 s. For subgroups I, 8 disks from each contaminant group were cleaned with Ivoclean, applied to the surface with a micro-brush, and left for 1 min. Each disk was then water-sprayed for 15 s and air-dried for 15 s.
Conditioning
In accordance with the clinical implication provided in a previous study,13 all specimens from all groups were conditioned before cementation with a MMA-based universal primer, visio.link (Bredent Group, Senden, Germany). The primer was rubbed into the air-abraded surfaces, using an applicator brush for 10 s. The surfaces of the specimens were then air-dried for 5 s for thinning the primer layer, and subsequently light-cured for 40 s, using a dental curing unit with an output of 1,200 mW/cm2 before cementation.
Bonding the specimens
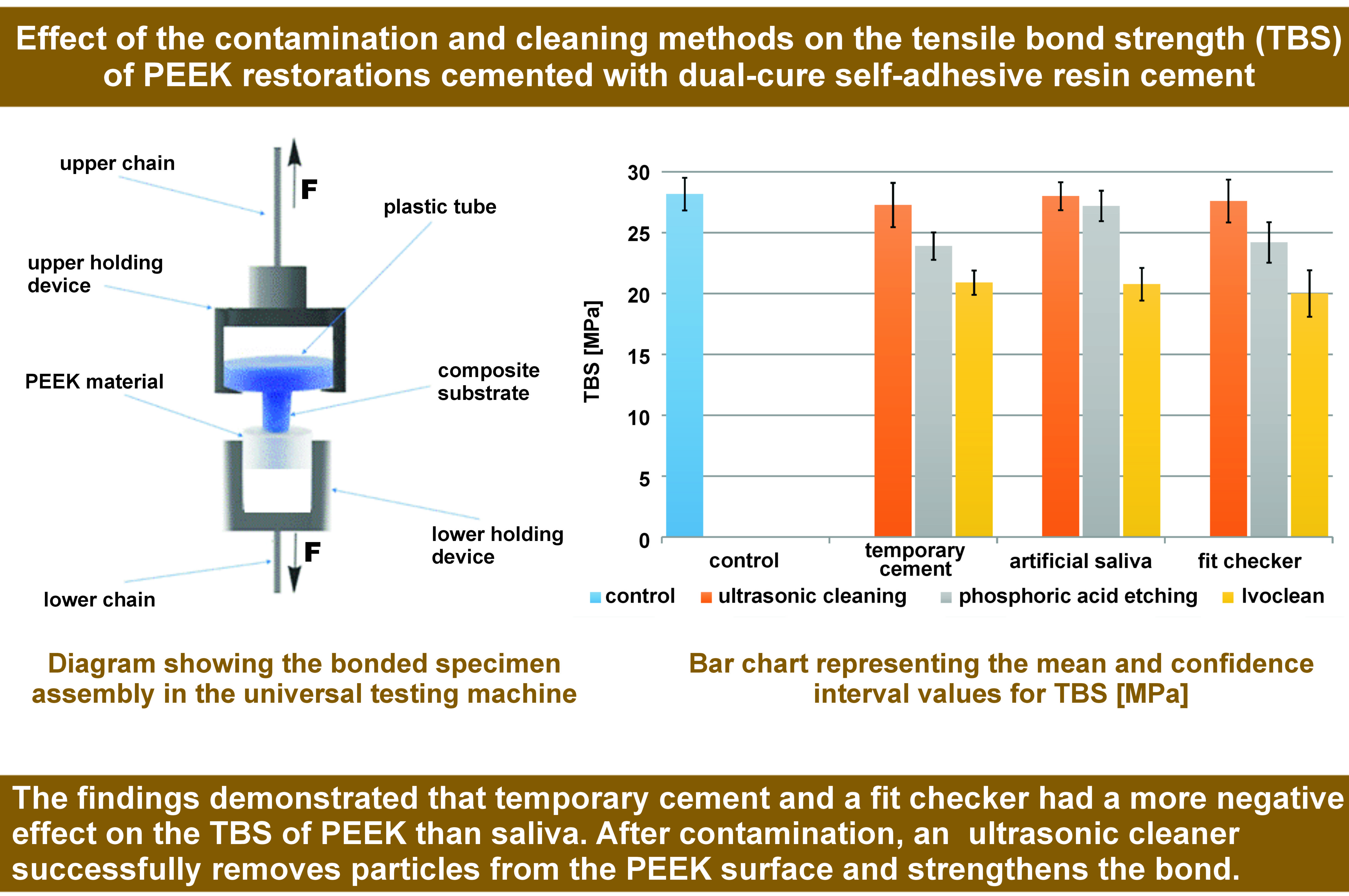
3D-printed plastic tubes (Formlab2; Formlabs Inc., Somerville, USA), with an inner diameter of 3.2 mm and a length of 10 mm, were fabricated and filled with a dual-cure composite resin (MultiCore Flow; Ivoclar Vivadent). Seven minutes after filling the tubes, they were bonded to the pretreated PEEK disks under a load of 2 kg with the use of dual-cure self-adhesive cement (RelyX™ Unicem; 3M ESPE). A separating agent was applied to the 1-millimeter plastic tube border around the dual-cure composite resin to avoid any interference with bonding. Excess cement was removed and air-blocking gel (Liquid Strip; Ivoclar Vivadent) was applied around the bonding margins to prevent the formation of an oxygen inhibition layer on the luting resin. Light polymerization of all specimens was performed from 2 opposing sites for 20 s at a light intensity of 1,200 mW/cm2.
Tensile bond strength testing
All specimens were stored in distilled water at 37°C for 24 h. This experimental study was carried out in accordance with the International Organization for Standardization (ISO) guidance ISO/TR 11405:1994.23 The specimens were loaded with a tensile force at a crosshead speed of 2 mm/min until failure with a universal testing machine (UTM) (Z010/TN2A; ZwickRoell, Ulm, Germany). The tensile bond strength was calculated with the following formula: fracture load / bonded area (N/mm2). Since the bonded area was constant in all specimens, TBS was automatically calculated by the SCM3000 testing software (MICROTEST, Madrid, Spain).
Failure mode analysis
The fractured interfaces of the specimens were examined under a digital microscope (Dino-Lite; AnMo Electronics Corp., New Taipei City, Taiwan) at ×40 magnification to determine the mode of failure. The failure modes were categorized into the following 3 types24: adhesive failure (type 1); cohesive failure in the luting resin or PEEK (type 2); and mixed failure, when one area exhibited cohesive failure, while other areas exhibited an adhesive fracture (type 3).
Statistical analysis
Numerical data was represented as mean and standard deviation (M ±SD) values. The Shapiro–Wilk test was used to check for normality. The homogeneity of variance was tested using Levene’s test. The data showed parametric distribution and variance homogeneity, and was analyzed using the two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. The comparisons of simple main effects were done utilizing the error term of the two-way model, with the p-value adjustment using the Bonferroni correction. The intergroup comparisons – to compare different groups with the control group – were done using the one-way ANOVA followed by Dunnett’s post hoc test. The significance level was set at p < 0.05 for all tests. Statistical analysis was performed with the R statistical analysis software for Windows, v. 4.1.3.25
Results
The results of the two-way ANOVA for the TBS values showed that there was a significant interaction between both tested variables (the contamination and cleaning methods) (p < 0.001). The comparisons of simple main effects showed that for the samples cleaned with an ultrasonic cleaner and Ivoclean, there were no significant differences with regard to the effects of the different contaminants (p > 0.05). However, for the samples treated with phosphoric acid, the difference was statistically significant (p = 0.005), and the post hoc pairwise comparisons showed the samples contaminated with artificial saliva to have a significantly higher mean TBS value than the samples subjected to other contaminants (p = 0.005) (Table 1).
For the samples contaminated with temporary cement and a fit checker, there were significant differences between the different cleaning methods, with ultrasonic cleaning providing the highest TBS values, followed by phosphoric acid etching, and finally Ivoclean, and with all pairwise comparisons being statistically significant (p < 0.001). For the samples contaminated with artificial saliva, the difference was also statistically significant (p < 0.001), but the pairwise comparisons showed only the samples cleaned with Ivoclean to have a significantly lower mean TBS value than other subgroups (p < 0.001) (Table 1).
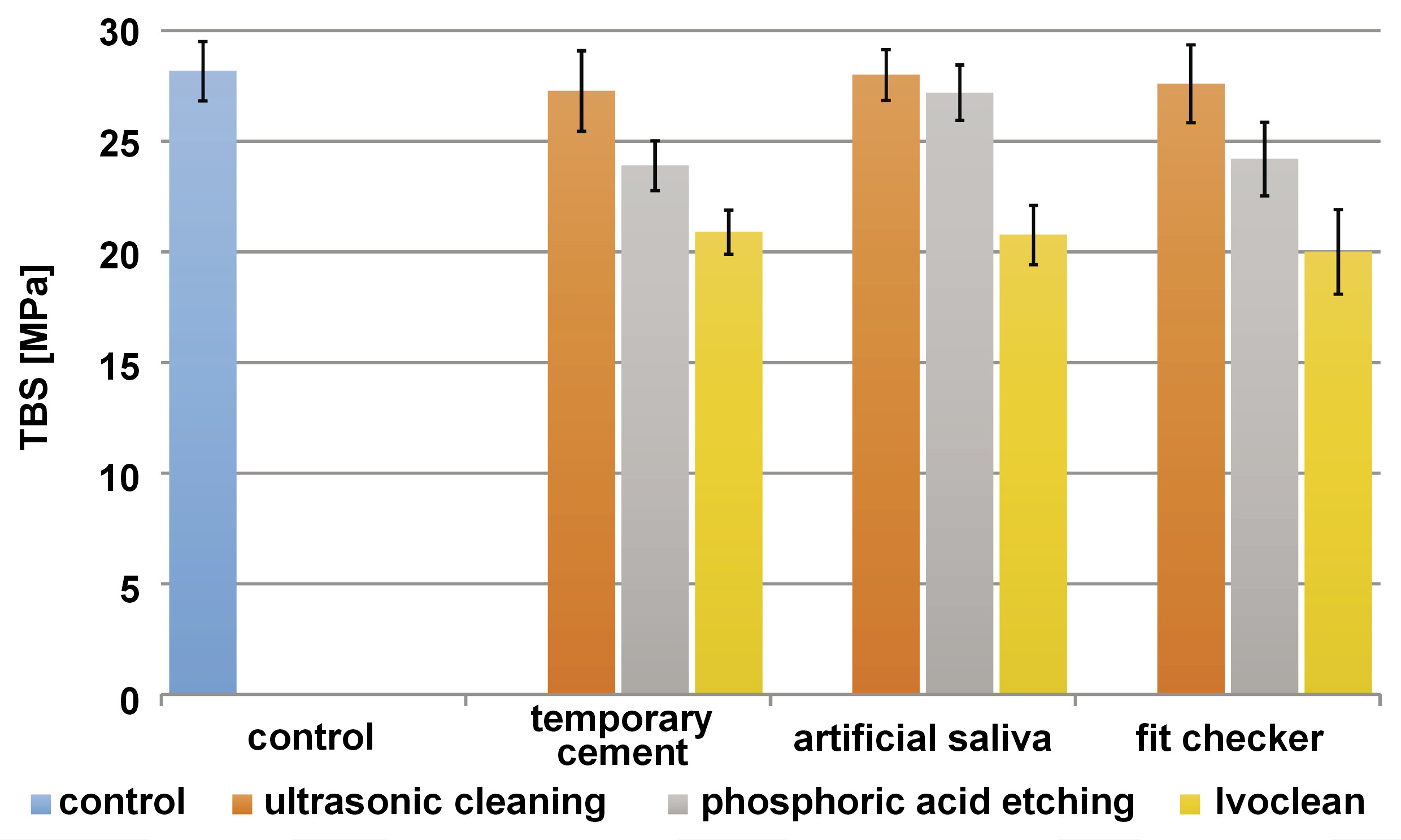
The results of the intergroup comparisons showed the samples contaminated with temporary cement and a fit checker, and treated with phosphoric acid, as well as all samples cleaned with Ivoclean, to be statistically significantly different from the control group (p < 0.001) (Table 2). The mean values with 95% confidence intervals (CIs) for TBS are presented in Figure 1.
Discussion
Contamination during the try-in procedure makes surface conditioning before the cementation of the restorative material difficult.17, 26, 27 In a previous study, artificial saliva, temporary cement and a silicone-disclosing medium were shown to decrease the bond strength of restorations.28 In the present study, eugenol-free temporary cement was used, since eugenol has been shown to significantly decrease the bond strength of restorative materials to resin cement.27 Also, artificial saliva was used, including mainly inorganic components, such as calcium (Ca) and phosphate, as well as proteins in the form of immunoglobulin and the salivary amylase enzyme, since the use of human saliva in experimental studies may lead to ethical concerns, or problems in the reproducibility and standardization of experiments due to human variation.29
The cleaning methods used in this study were chosen according to their availability in the clinic as a chairside procedure and due to their presence in the literature as methods for cleaning contamination off the surface of the restorative material to be cemented. Previous studies reported different cleaning methods, both mechanical and chemical, to improve the bond strength of the contaminated surfaces.30, 31 The use of phosphoric acid was reported by Aboush to be the most beneficial method to remove saliva from the contaminated porcelain veneer surface.15 Wattanasirmkit and Charasseangpaisarn stated that the saliva and silicone-disclosing medium contaminating zirconia should be cleaned with phosphoric acid, Ivoclean or hydrofluoric acid for 20 s prior to cementation.32 In a study by Phark et al., combining the ultrasonic cleaner isopropanol bath and phosphoric acid proved to be an efficient method for cleaning the contaminated zirconia surfaces.33 The bond strength of resin cement to the saliva-contaminated lithium disilicate ceramic etched with hydrofluoric acid was proven by Yoshida to be restored when cleaned with phosphoric acid and the Ivoclean gel.34
The grouping system in this study was applied in accordance with other previous studies measuring the TBS of different materials.35, 36 The bonding protocol used was based on a previous study by Kern and Lehmann, who concluded that durable bonding to PEEK could be achieved using a multifunctional methacrylate-containing resin varnish on the air-abraded PEEK surfaces.12 The visio.link primer has a good wetting ability that allows good mechanical interlocking in the micropores of the surface. It contains MMA and a highly reactive triacrylate monomer, pentaerythritol triacrylate (PETIA), which can penetrate the resin matrix of the polymeric restoration material and create entanglements that function as mechanical connections. Furthermore, visio.link allows covalent bonding to methacrylate in polymeric restoration materials and resin composite cement, providing high crosslinking density at the interface and good mechanical properties.37
The null hypothesis was rejected, since the contamination and cleaning methods had a significant effect on the TBS between PEEK and dual-cure self-adhesive resin cement.
The results of the current study showed that contamination with temporary cement and a fit checker had the greatest effect on the TBS of PEEK cemented with resin when cleaned with phosphoric acid and Ivoclean. The difference compared with the control group was significant. Residues may affect the wettability of the adherend surface with the self-adhesive resin cement, and act as a barrier that inhibits the interactions between the inorganic fillers of the self-adhesive resin cement and the organic monomers in the polymer matrix.27, 38 Ultrasonic cleaning was the most effective cleaning method to remove all 3 contaminants. Güers et al. found that cleaning was efficient with combining mechanical and chemical cleaning methods, and that isopropanol was an excellent solvent and left almost no oil traces on the surface.20 Other studies have found that an ultrasonic bath triggers the release of larger particles, as well as some sub-micron particles, significantly reducing the amount of contaminant left on the adherend surface.39
Contamination with artificial saliva had the least significant effect on the TBS of PEEK cemented with resin, and the difference compared with the control group was not significant except for the combination with Ivoclean. This can be explained by the fact that PEEK is a polymer with which artificial saliva does not react chemically, as it has no outer oxide layer that can be infiltrated by saliva; it merely affects its hydrophilicity. Unlike zirconia, where artificial saliva significantly affects its TBS to resin cement due to the phospholipids present in saliva that bond to and occupy the outer oxide layer of zirconia, leaving little remaining oxide layer space that visio.link could bond to.29
There was a slight interaction between artificial saliva and PEEK, which was easily diminished by phosphoric acid. It is possible that the acid penetrates the salivary film and etches the adherend surface underneath it.15 Regarding Ivoclean, its composition is specially designed to remove saliva contamination. The zirconium oxide particles in Ivoclean can strongly interact with the phosphate groups in the salivary film and remove the film from the adherent surface.29
This study used a tensile test to evaluate the adhesive capacity of the material rather than, for instance, the stress created during clinical function. However, intraorally, indirect restorations are subjected to different forces, such as tensile, shear, compressive, oblique, and combinations of these types. This study evaluated only tensile forces. The interpretation of the current TBS results is that the different contaminants affected the bond strength differently, depending on the cleaning method.
The results of this research are in agreement with previous papers studying different restorative materials other than PEEK under similar conditions. Temporary cement and a fit checker affected the TBS of PEEK more than artificial saliva, and the ultrasonic isopropanol cleaner bath had a better cleaning effect than phosphoric acid or Ivoclean. In this article, TBS was measured in vitro, without thermal or mechanical load cycling. The experiments involving thermocycling and mechanical loading provide a better evaluation of the bond strength, so we recommend more research on the long-term stability of the bond strength, using different aging protocols.
Conclusions
Within the limitations of this study, the following conclusions could be drawn:
Temporary cement and a fit checker adversely affected the TBS of PEEK, more than artificial saliva.
An ultrasonic cleaner effectively decontaminates the PEEK surface and enhances the TBS to resin cement.
Ethics approval and consent to participate
Not applicable.
Data availability
The datasets supporting the findings of the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.